Total Synthesis of (+)-Rubriflordilactone A
- PMID: 26337920
- PMCID: PMC4643188
- DOI: 10.1002/anie.201506366
Total Synthesis of (+)-Rubriflordilactone A
Abstract
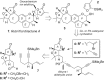
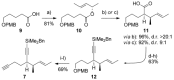
Two enantioselective total syntheses of the nortriterpenoid natural product rubriflordilactone A are described, which use palladium- or cobalt-catalyzed cyclizations to form the CDE rings, and converge on a late-stage synthetic intermediate. These key processes are set up through the convergent coupling of a common diyne component with appropriate AB-ring aldehydes, a strategy that sets the stage for the synthetic exploration of other members of this family of natural products.
Keywords: cyclotrimerization; domino reactions; natural products; total synthesis; transition metal catalysis.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures



References
-
- Shi Y-M, Xiao W-L, Pu J-X, Sun H-D. Nat. Prod. Rep. 2015;32:367. Reviews on Schisandraceae natural products. - PubMed
-
- Xia Y-G, Yang B-Y, Kuang H-X. Phytochem. Rev. 2015;14:155. For reviews, see.
-
- Xiao WL, Li RT, Huang SX, Pu JX, Sun HD. Nat. Prod. Rep. 2008;25:871. - PubMed
-
- Xiao Q, Ren WW, Chen ZX, Sun TW, Li Y, Ye QD, Gong JX, Meng FK, You L, Liu YF, Zhao MZ, Xu LM, Shan ZH, Shi Y, Tang YF, Chen JH, Yang Z. Angew. Chem. Int. Ed. 2011;50:7373. - PubMed
-
- Angew. Chem. 2011;123:7511.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

