Bronchoabsorption; a novel bronchoscopic technique to improve biomarker sampling of the airway
- PMID: 26338015
- PMCID: PMC4559920
- DOI: 10.1186/s12931-015-0268-5
Bronchoabsorption; a novel bronchoscopic technique to improve biomarker sampling of the airway
Abstract
Background: Current techniques used to obtain lung samples have significant limitations and do not provide reproducible biomarkers of inflammation. We have developed a novel technique that allows multiple sampling methods from the same area (or multiple areas) of the lung under direct bronchoscopic vision. It allows collection of mucosal lining fluid and bronchial brushing from the same site; biopsy samples may also be taken. The novel technique takes the same time as standard procedures and can be conducted safely.
Methods: Eight healthy smokers aged 40-65 years were included in this study. An absorptive filter paper was applied to the bronchial mucosa under direct vision using standard bronchoscopic techniques. Further samples were obtained from the same site using bronchial brushings. Bronchoalveolar lavage (BAL) was obtained using standard techniques. Chemokine (C-C Motif) Ligand 20 (CCL20), CCL4, CCL5, Chemokine (C-X-C Motif) Ligand 1 (CXCL1), CXCL8, CXCL9, CXCL10, CXCL11, Interleukin 1 beta (IL-1β), IL-6, Vascular endothelial growth factor (VEGF), Matrix metalloproteinase 8 (MMP-8) and MMP-9 were measured in exudate and BAL. mRNA was collected from the bronchial brushings for gene expression analysis.
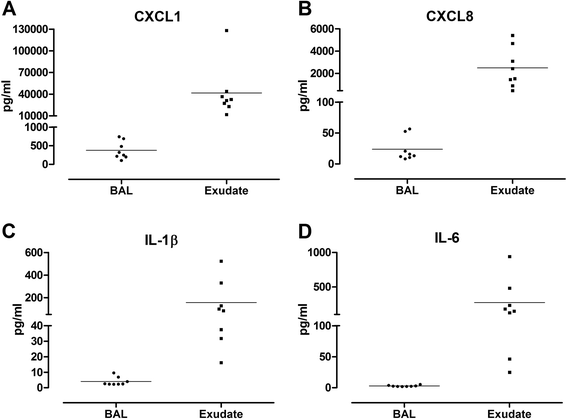
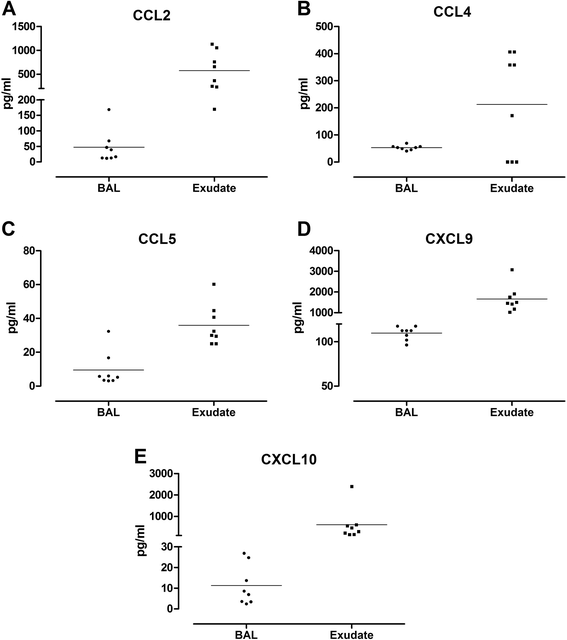
Results: A greater than 10 fold concentration of all the biomarkers was detected in lung exudate in comparison to BAL. High yield of good quality RNA with RNA integrity numbers (RIN) between 7.6 and 9.3 were extracted from the bronchial brushings. The subset of genes measured were reproducible across the samples and corresponded to the inflammatory markers measured in exudate and BAL.
Conclusions: The bronchoabsorption technique as described offers the ability to sample lung fluid direct from the site of interest without the dilution effects caused by BAL. Using this method we were able to successfully measure the concentrations of biomarkers present in the lungs as well as collect high yield mRNA samples for gene expression analysis from the same site. This technique demonstrates superior sensitivity to standard BAL for the measurement of biomarkers of inflammation. It could replace BAL as the method of choice for these measurements. This method provides a systems biology approach to studying the inflammatory markers of respiratory disease progression.
Trial registration: NHS Health Research Authority (13/LO/0256).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

