Haste Makes Waste: The Interplay Between Dissolution and Precipitation of Supersaturating Formulations
- PMID: 26338234
- PMCID: PMC4627464
- DOI: 10.1208/s12248-015-9825-6
Haste Makes Waste: The Interplay Between Dissolution and Precipitation of Supersaturating Formulations
Abstract
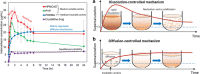
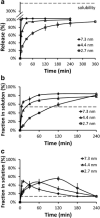
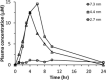
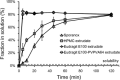
Contrary to the early philosophy of supersaturating formulation design for oral solid dosage forms, current evidence shows that an exceedingly high rate of supersaturation generation could result in a suboptimal in vitro dissolution profile and subsequently could reduce the in vivo oral bioavailability of amorphous solid dispersions. In this commentary, we outline recent research efforts on the specific effects of the rate and extent of supersaturation generation on the overall kinetic solubility profiles of supersaturating formulations. Additional insights into an appropriate definition of sink versus nonsink dissolution conditions and the solubility advantage of amorphous pharmaceuticals are also highlighted. The interplay between dissolution and precipitation kinetics should be carefully considered in designing a suitable supersaturating formulation to best improve the dissolution behavior and oral bioavailability of poorly water-soluble drugs.
Keywords: amorphous formulation; kinetic solubility; nonsink dissolution testing; poorly water-soluble drug; supersaturation rate.
Figures



References
-
- Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19(12):930–4. doi: 10.1021/ja02086a003. - DOI
-
- Nernst W. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Zeitschrift. 1904;47:52–5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

