Moving and positioning the nucleus in skeletal muscle - one step at a time
- PMID: 26338260
- PMCID: PMC4915500
- DOI: 10.1080/19491034.2015.1090073
Moving and positioning the nucleus in skeletal muscle - one step at a time
Abstract
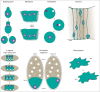
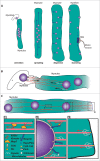
Nuclear movement and positioning within cells has become an area of great interest in the past few years due to the identification of different molecular mechanisms and functions in distinct organisms and contexts. One extreme example occurs during skeletal muscle development and regeneration. Skeletal muscles are composed of individual multinucleated myofibers with nuclei positioned at their periphery. Myofibers are formed by fusion of mononucleated myoblasts and during their development, successive nuclear movements and positioning events have been described. The position of the nuclei in myofibers is important for muscle function. Interestingly, during muscle regeneration and in some muscular diseases, nuclei are positioned in the center of the myofiber. In this review, we discuss the multiple mechanisms of nuclear positioning that occur during myofiber formation and regeneration. We also discuss the role of nuclear positioning for skeletal muscle function.
Keywords: Nuclear movement; cytoskeleton; nuclear envelope; skeletal muscle.
Figures
References
-
- Osorio DS, Gomes ER. The contemporary nucleus: A trip down memory lane. Biol Cell 2013; 105:430-41; PMID:23802772; http://dx.doi.org/10.1111/boc.201300009 - DOI - PubMed
-
- Sauer F. Mitosis in the neural tube. J Comp Neurol 1935; 62:377-405; http://dx.doi.org/10.1002/cne.900620207 - DOI
-
- Gundersen GG, Worman HJ. Nuclear Positioning. Cell 2013; 152:1376-89; PMID:23498944; http://dx.doi.org/10.1016/j.cell.2013.02.031 - DOI - PMC - PubMed
-
- Wilson EB. The cell in development and heredity [Internet]. MacMillan Publishing Co. 1928. [cited 2013 Aug 21] Available from: http://wellcomelibrary.org/player/b18022832
-
- Lee W-L OJ. The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J Cell Biol 2003; 160:355-64; PMID:12566428; http://dx.doi.org/10.1083/jcb.200209022 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
