Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Aβ Generation and Amyloid Plaque Pathogenesis
- PMID: 26338325
- PMCID: PMC4556784
- DOI: 10.1523/JNEUROSCI.0705-15.2015
Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Aβ Generation and Amyloid Plaque Pathogenesis
Abstract
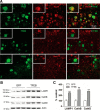
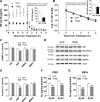
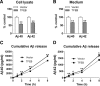
In AD, an imbalance between Aβ production and removal drives elevated brain Aβ levels and eventual amyloid plaque deposition. APP undergoes nonamyloidogenic processing via α-cleavage at the plasma membrane, amyloidogenic β- and γ-cleavage within endosomes to generate Aβ, or lysosomal degradation in neurons. Considering multiple reports implicating impaired lysosome function as a driver of increased amyloidogenic processing of APP, we explored the efficacy of targeting transcription factor EB (TFEB), a master regulator of lysosomal pathways, to reduce Aβ levels. CMV promoter-driven TFEB, transduced via stereotactic hippocampal injections of adeno-associated virus particles in APP/PS1 mice, localized primarily to neuronal nuclei and upregulated lysosome biogenesis. This resulted in reduction of APP protein, the α and β C-terminal APP fragments (CTFs), and in the steady-state Aβ levels in the brain interstitial fluid. In aged mice, total Aβ levels and amyloid plaque load were selectively reduced in the TFEB-transduced hippocampi. TFEB transfection in N2a cells stably expressing APP695, stimulated lysosome biogenesis, reduced steady-state levels of APP and α- and β-CTFs, and attenuated Aβ generation by accelerating flux through the endosome-lysosome pathway. Cycloheximide chase assays revealed a shortening of APP half-life with exogenous TFEB expression, which was prevented by concomitant inhibition of lysosomal acidification. These data indicate that TFEB enhances flux through lysosomal degradative pathways to induce APP degradation and reduce Aβ generation. Activation of TFEB in neurons is an effective strategy to attenuate Aβ generation and attenuate amyloid plaque deposition in AD.
Significance statement: A key driver for AD pathogenesis is the net balance between production and clearance of Aβ, the major component of amyloid plaques. Here we demonstrate that lysosomal degradation of holo-APP influences Aβ production by limiting the availability of APP for amyloidogenic processing. Using viral gene transfer of transcription factor EB (TFEB), a master regulator of lysosome biogenesis in neurons of APP/PS1 mice, steady-state levels of APP were reduced, resulting in decreased interstitial fluid Aβ levels and attenuated amyloid deposits. These effects were caused by accelerated lysosomal degradation of endocytosed APP, reflected by reduced APP half-life and steady-state levels in TFEB-expressing cells, with resultant decrease in Aβ production and release. Additional studies are needed to explore the therapeutic potential of this approach.
Keywords: Alzheimer's disease; TFEB; amyloid; amyloid precursor protein; lysosome.
Copyright © 2015 the authors 0270-6474/15/3512137-15$15.00/0.
Figures


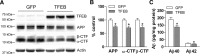


References
-
- Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem. 2013;288:1295–1306. doi: 10.1074/jbc.M112.409250. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
