Low-voltage paper isotachophoresis device for DNA focusing
- PMID: 26338530
- PMCID: PMC4589534
- DOI: 10.1039/c5lc00875a
Low-voltage paper isotachophoresis device for DNA focusing
Abstract
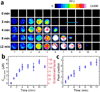
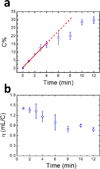
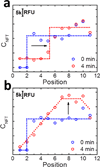
We present a new paper-based isotachophoresis (ITP) device design for focusing DNA samples having lengths ranging from 23 to at least 1517 bp. DNA is concentrated by more than two orders of magnitude within 4 min. The key component of this device is a 2 mm-long, 2 mm-wide circular paper channel formed by concertina folding a paper strip and aligning the circular paper zones on each layer. Due to the short channel length, a high electric field of ~16 kV m(-1) is easily generated in the paper channel using two 9 V batteries. The multilayer architecture also enables convenient reclamation and analysis of the sample after ITP focusing by simply opening the origami paper and cutting out the desired layers. We profiled the electric field in the origami paper channel during ITP experiments using a nonfocusing fluorescent tracer. The result showed that focusing relied on formation and subsequent movement of a sharp electric field boundary between the leading and trailing electrolyte.
Figures



References
-
- Jung B, Bharadwaj R, Santiago JG. On-chip millionfold sample stacking using transient isotachophoresis. Anal. Chem. 2006;78:2319–2327. - PubMed
-
- Liu H, Crooks RM. Three-Dimensional Paper Microfluidic Devices Assembled Using the Principles of Origami. J. Am. Chem. Soc. 2011;133:17564–17566. - PubMed
-
- Luo L, Li X, Crooks RM. Low-Voltage Origami-Paper-Based Electrophoretic Device for Rapid Protein Separation. Anal. Chem. 2014;86:12390–12397. - PubMed
-
- Carrilho E, Martinez AW, Whitesides GM. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009;81:7091–7095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

