Exposure to airborne PM2.5 suppresses microRNA expression and deregulates target oncogenes that cause neoplastic transformation in NIH3T3 cells
- PMID: 26338969
- PMCID: PMC4745737
- DOI: 10.18632/oncotarget.5005
Exposure to airborne PM2.5 suppresses microRNA expression and deregulates target oncogenes that cause neoplastic transformation in NIH3T3 cells
Abstract
Long-term exposure to airborne PM2.5 is associated with increased lung cancer risk but the underlying mechanism remains unclear. We characterized global microRNA and mRNA expression in human bronchial epithelial cells exposed to PM2.5 organic extract and integrally analyzed microRNA-mRNA interactions. Foci formation and xenograft tumorigenesis in mice with NIH3T3 cells expressing genes targeted by microRNAs were performed to explore the oncogenic potential of these genes. We also detected plasma levels of candidate microRNAs in subjects exposed to different levels of air PM2.5 and examined the aberrant expression of genes targeted by these microRNAs in human lung cancer. Under our experimental conditions, treatment of cells with PM2.5 extract resulted in downregulation of 138 microRNAs and aberrant expression of 13 mRNAs (11 upregulation and 2 downregulation). In silico and biochemical analyses suggested SLC30A1, SERPINB2 and AKR1C1, among the upregulated genes, as target for miR-182 and miR-185, respectively. Ectopic expression of each of these genes significantly enhanced foci formation in NIH3T3 cells. Following subcutaneous injection of these cells into nude mice, fibrosarcoma were formed from SLC30A1- or SERPINB2-expressing cells. Reduced plasma levels of miR-182 were detected in subjects exposed to high level of PM2.5 than in those exposed to low level of PM2.5 (P = 0.043). Similar results were seen for miR-185 although the difference was not statistically significant (P = 0.328). Increased expressions of SLC30A1, SERPINB2 and AKR1C1 were detected in human lung cancer. These results suggest that modulation of miR-182 and miR-185 and their target genes may contribute to lung carcinogenesis attributable to PM2.5 exposure.
Keywords: PM2.5; bronchial epithelial cell; gene expression; microRNA expression; neoplastic transformation.
Conflict of interest statement
No conflict financial interests exist.
Figures

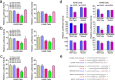
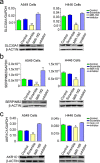



References
-
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. - PubMed
-
- Bandowe BA, Meusel H, Huang RJ, Ho K, Cao J, Hoffmann T, Wilcke W. PM(2).(5)-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: seasonal variation, sources and cancer risk assessment. Sci Total Environ. 2014:473–474. 77–87. - PubMed
-
- Wang JL, Zhang YH, Shao M, Liu XL, Zeng LM, Cheng CL, Xu XF. Chemical composition and quantitative relationship between meteorological condition and fine particles in Beijing. J Environ Sci (China) 2004;16:860–864. - PubMed
-
- Leung PY, Wan HT, Billah MB, Cao JJ, Ho KF, Wong CK. Chemical and biological characterization of air particulate matter 2.5, collected from five cities in China. Environ Pollut. 2014;194:188–195. - PubMed
-
- Li J, Wang G, Aggarwal SG, Huang Y, Ren Y, Zhou B, Singh K, Gupta PK, Cao J, Zhang R. Comparison of abundances, compositions and sources of elements, inorganic ions and organic compounds in atmospheric aerosols from Xi'an and New Delhi, two megacities in China and India. Sci Total Environ. 2014:476–477. 485–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

