Structural Basis of the Inhibition of STAT1 Activity by Sendai Virus C Protein
- PMID: 26339056
- PMCID: PMC4645678
- DOI: 10.1128/JVI.01887-15
Structural Basis of the Inhibition of STAT1 Activity by Sendai Virus C Protein
Abstract
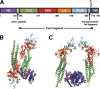
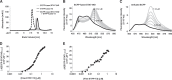
Sendai virus (SeV) C protein inhibits the signal transduction pathways of interferon alpha/beta (IFN-α/β) and IFN-γ by binding to the N-terminal domain of STAT1 (STAT1ND), thereby allowing SeV to escape from host innate immunity. Here we determined the crystal structure of STAT1ND associated with the C-terminal half of the C protein (Y3 [amino acids 99 to 204]) at a resolution of 2.0 Å. This showed that two molecules of Y3 symmetrically bind to each niche created between two molecules of the STAT1ND dimer. Molecular modeling suggested that an antiparallel form of the full-length STAT1 dimer can bind only one Y3 molecule and that a parallel form can bind two Y3 molecules. Affinity analysis demonstrated anticooperative binding of two Y3 molecules with the STAT1 dimer, which is consistent with the hypothetical model that the second Y3 molecule can only target the STAT1 dimer in a parallel form. STAT1 with excess amounts of Y3 was prone to inhibit the dephosphorylation at Tyr(701) by a phosphatase. In an electrophoretic mobility shift assay, tyrosine-phosphorylated STAT1 (pY-STAT1) with Y3 associated with the γ-activated sequence, probably as high-molecular-weight complexes (HMWCs), which may account for partial inhibition of a reporter assay from IFN-γ by Y3. Our study suggests that the full-length C protein interferes with the domain arrangement of the STAT1 dimer, leading to the accumulation of pY-STAT1 and the formation of HMWCs. In addition, we discuss the mechanism by which phosphorylation of STAT2 is inhibited in the presence of the C protein after stimulation by IFN-α/β.
Importance: Sendai virus, a paramyxovirus that causes respiratory diseases in rodents, possesses the C protein, which inhibits the signal transduction pathways of interferon alpha/beta (IFN-α/β) and IFN-γ by binding to the transcription factor STAT1. In virus-infected cells, phosphorylation of STAT1 at the Tyr(701) residue is potently enhanced, although transcription by STAT1 is inert. Here, we determined the crystal structure of the N-terminal domain of STAT1 associated with the C-terminal half of the C protein. Molecular modeling and experiments suggested that the two C proteins bind to and stabilize the parallel form of the STAT1 dimer, which are likely to be phosphorylated at Tyr(701), further inducing high-molecular-weight complex formation and inhibition of transcription by IFN-γ. We also discuss the possible mechanism of inhibition of the IFN-α/β pathways by the C protein. This is the first structural report of the C protein, suggesting a mechanism of evasion of the paramyxovirus from innate immunity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures








Similar articles
-
Structural analysis of the STAT1:STAT2 heterodimer revealed the mechanism of Sendai virus C protein-mediated blockade of type 1 interferon signaling.J Biol Chem. 2017 Dec 1;292(48):19752-19766. doi: 10.1074/jbc.M117.786285. Epub 2017 Oct 4. J Biol Chem. 2017. PMID: 28978648 Free PMC article.
-
The STAT2 activation process is a crucial target of Sendai virus C protein for the blockade of alpha interferon signaling.J Virol. 2003 Mar;77(6):3360-70. doi: 10.1128/jvi.77.6.3360-3370.2003. J Virol. 2003. PMID: 12610111 Free PMC article.
-
Inhibition of IFN-alpha/beta signaling by two discrete peptides within measles virus V protein that specifically bind STAT1 and STAT2.Virology. 2009 Jan 5;383(1):112-20. doi: 10.1016/j.virol.2008.10.014. Epub 2008 Nov 12. Virology. 2009. PMID: 19007958
-
[Sendai virus proteins counteracting the host innate immunity].Uirusu. 2004 Dec;54(2):179-88. doi: 10.2222/jsv.54.179. Uirusu. 2004. PMID: 15745155 Review. Japanese.
-
STAT1 and pathogens, not a friendly relationship.Biochimie. 2010 May;92(5):425-44. doi: 10.1016/j.biochi.2010.02.009. Epub 2010 Feb 13. Biochimie. 2010. PMID: 20159032 Free PMC article. Review.
Cited by
-
The Dual Role of STAT1 in Ovarian Cancer: Insight Into Molecular Mechanisms and Application Potentials.Front Cell Dev Biol. 2021 Mar 23;9:636595. doi: 10.3389/fcell.2021.636595. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33834023 Free PMC article. Review.
-
C Proteins: Controllers of Orderly Paramyxovirus Replication and of the Innate Immune Response.Viruses. 2022 Jan 12;14(1):137. doi: 10.3390/v14010137. Viruses. 2022. PMID: 35062341 Free PMC article. Review.
-
MGF360-9L Is a Major Virulence Factor Associated with the African Swine Fever Virus by Antagonizing the JAK/STAT Signaling Pathway.mBio. 2022 Feb 22;13(1):e0233021. doi: 10.1128/mbio.02330-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35076286 Free PMC article.
-
Viral Inhibition of the IFN-Induced JAK/STAT Signalling Pathway: Development of Live Attenuated Vaccines by Mutation of Viral-Encoded IFN-Antagonists.Vaccines (Basel). 2016 Jun 29;4(3):23. doi: 10.3390/vaccines4030023. Vaccines (Basel). 2016. PMID: 27367734 Free PMC article. Review.
-
Molecular Mechanisms of Innate Immune Inhibition by Non-Segmented Negative-Sense RNA Viruses.J Mol Biol. 2016 Aug 28;428(17):3467-82. doi: 10.1016/j.jmb.2016.07.017. Epub 2016 Jul 31. J Mol Biol. 2016. PMID: 27487481 Free PMC article. Review.
References
-
- Lamb RA, Parks GD. 2013. Paramyxoviridae: the viruses and their replication, p 957–995. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Nagai Y, Takakura A, Irie T, Yonemitsu Y, Gotoh B. 2011. Sendai virus: evolution from mouse pathogen to a state-of-the-art tool in virus research and biotechnology, p 115–173. In Samal SK. (ed), The biology of paramyxoviruses. Caister Academic Press, Norfolk, United Kingdom.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

