Validation of the 2nd Generation Proteasome Inhibitor Oprozomib for Local Therapy of Pulmonary Fibrosis
- PMID: 26340365
- PMCID: PMC4560391
- DOI: 10.1371/journal.pone.0136188
Validation of the 2nd Generation Proteasome Inhibitor Oprozomib for Local Therapy of Pulmonary Fibrosis
Abstract
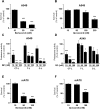
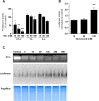
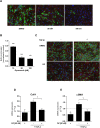
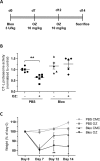
Proteasome inhibition has been shown to prevent development of fibrosis in several organs including the lung. However, effects of proteasome inhibitors on lung fibrosis are controversial and cytotoxic side effects of the overall inhibition of proteasomal protein degradation cannot be excluded. Therefore, we hypothesized that local lung-specific application of a novel, selective proteasome inhibitor, oprozomib (OZ), provides antifibrotic effects without systemic toxicity in a mouse model of lung fibrosis. Oprozomib was first tested on the human alveolar epithelial cancer cell line A549 and in primary mouse alveolar epithelial type II cells regarding its cytotoxic effects on alveolar epithelial cells and compared to the FDA approved proteasome inhibitor bortezomib (BZ). OZ was less toxic than BZ and provided high selectivity for the chymotrypsin-like active site of the proteasome. In primary mouse lung fibroblasts, OZ showed significant anti-fibrotic effects, i.e. reduction of collagen I and α smooth muscle actin expression, in the absence of cytotoxicity. When applied locally into the lungs of healthy mice via instillation, OZ was well tolerated and effectively reduced proteasome activity in the lungs. In bleomycin challenged mice, however, locally applied OZ resulted in accelerated weight loss and increased mortality of treated mice. Further, OZ failed to reduce fibrosis in these mice. While upon systemic application OZ was well tolerated in healthy mice, it rather augmented instead of attenuated fibrotic remodelling of the lung in bleomycin challenged mice. To conclude, low toxicity and antifibrotic effects of OZ in pulmonary fibroblasts could not be confirmed for pulmonary fibrosis of bleomycin-treated mice. In light of these data, the use of proteasome inhibitors as therapeutic agents for the treatment of fibrotic lung diseases should thus be considered with caution.
Conflict of interest statement
Figures


Similar articles
-
In vivo investigations on anti-fibrotic potential of proteasome inhibition in lung and skin fibrosis.Am J Respir Cell Mol Biol. 2008 Oct;39(4):458-65. doi: 10.1165/rcmb.2007-0320OC. Epub 2008 May 5. Am J Respir Cell Mol Biol. 2008. PMID: 18458239
-
Histone deacetylase inhibitor restores surfactant protein-C expression in alveolar-epithelial type II cells and attenuates bleomycin-induced pulmonary fibrosis in vivo.Exp Lung Res. 2015;41(8):422-34. doi: 10.3109/01902148.2015.1060275. Epub 2015 Jul 7. Exp Lung Res. 2015. PMID: 26151196
-
Dual inhibition of αvβ6 and αvβ1 reduces fibrogenesis in lung tissue explants from patients with IPF.Respir Res. 2021 Oct 19;22(1):265. doi: 10.1186/s12931-021-01863-0. Respir Res. 2021. PMID: 34666752 Free PMC article.
-
Mimosa pudica L. extract ameliorates pulmonary fibrosis via modulation of MAPK signaling pathways and FOXO3 stabilization.J Ethnopharmacol. 2024 Aug 10;330:118226. doi: 10.1016/j.jep.2024.118226. Epub 2024 Apr 25. J Ethnopharmacol. 2024. PMID: 38670401
-
Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen.Arthritis Rheum. 2009 Jul;60(7):2142-55. doi: 10.1002/art.24620. Arthritis Rheum. 2009. PMID: 19565505
Cited by
-
Proteasome dysfunction in alveolar type 2 epithelial cells is associated with acute respiratory distress syndrome.Sci Rep. 2019 Aug 29;9(1):12509. doi: 10.1038/s41598-019-49020-4. Sci Rep. 2019. PMID: 31467330 Free PMC article.
-
The ubiquitin proteasome system as a potential therapeutic target for systemic sclerosis.Transl Res. 2018 Aug;198:17-28. doi: 10.1016/j.trsl.2018.03.003. Epub 2018 Mar 29. Transl Res. 2018. PMID: 29702079 Free PMC article. Review.
-
Bortezomib Inhibits Lung Fibrosis and Fibroblast Activation without Proteasome Inhibition.Am J Respir Cell Mol Biol. 2022 Jan;66(1):23-37. doi: 10.1165/rcmb.2021-0112OC. Am J Respir Cell Mol Biol. 2022. PMID: 34236953 Free PMC article.
-
Evaluation of Proteasome Inhibitors in the Treatment of Idiopathic Pulmonary Fibrosis.Cells. 2022 May 4;11(9):1543. doi: 10.3390/cells11091543. Cells. 2022. PMID: 35563849 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

