Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications
- PMID: 26340621
- PMCID: PMC4613230
- DOI: 10.3390/ijms160920774
Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications
Abstract
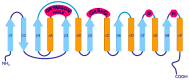
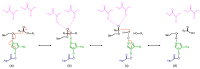
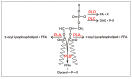
Lipases and phospholipases are interfacial enzymes that hydrolyze hydrophobic ester linkages of triacylglycerols and phospholipids, respectively. In addition to their role as esterases, these enzymes catalyze a plethora of other reactions; indeed, lipases also catalyze esterification, transesterification and interesterification reactions, and phospholipases also show acyltransferase, transacylase and transphosphatidylation activities. Thus, lipases and phospholipases represent versatile biocatalysts that are widely used in various industrial applications, such as for biodiesels, food, nutraceuticals, oil degumming and detergents; minor applications also include bioremediation, agriculture, cosmetics, leather and paper industries. These enzymes are ubiquitous in most living organisms, across animals, plants, yeasts, fungi and bacteria. For their greater availability and their ease of production, microbial lipases and phospholipases are preferred to those derived from animals and plants. Nevertheless, traditional purification strategies from microbe cultures have a number of disadvantages, which include non-reproducibility and low yields. Moreover, native microbial enzymes are not always suitable for biocatalytic processes. The development of molecular techniques for the production of recombinant heterologous proteins in a host system has overcome these constraints, as this allows high-level protein expression and production of new redesigned enzymes with improved catalytic properties. These can meet the requirements of specific industrial process better than the native enzymes. The purpose of this review is to give an overview of the structural and functional features of lipases and phospholipases, to describe the recent advances in optimization of the production of recombinant lipases and phospholipases, and to summarize the information available relating to their major applications in industrial processes.
Keywords: : lipases; biocatalysis; heterologous expression; industrial applications; phospholipases; protein engineering.
Figures


References
-
- Tyler J., Simurdiak M.R., Zhao H. Biocatalysis. In: Lee S., editor. Encyclopedia of Chemical Processing. Volume 1. CRC Press; London, UK: 2006. pp. 101–110.
-
- Lefevre F., Jarrin C., Ginolhac A., Auriol D., Nalin R. Environmental metagenomics: An innovative resource for industrial biocatalysis. Biocatal. Biotransform. 2007;25:242–250. doi: 10.1080/10242420701444314. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

