Optimization and Evaluation of a Chitosan/Hydroxypropyl Methylcellulose Hydrogel Containing Toluidine Blue O for Antimicrobial Photodynamic Inactivation
- PMID: 26340623
- PMCID: PMC4613232
- DOI: 10.3390/ijms160920859
Optimization and Evaluation of a Chitosan/Hydroxypropyl Methylcellulose Hydrogel Containing Toluidine Blue O for Antimicrobial Photodynamic Inactivation
Abstract
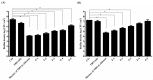
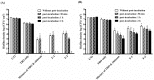
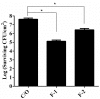
Photodynamic inactivation (PDI) combined with chitosan has been shown as a promising antimicrobial approach. The purpose of this study was to develop a chitosan hydrogel containing hydroxypropyl methylcellulose (HPMC), chitosan and toluidine blue O (TBO) to improve the bactericidal efficacy for topical application in clinics. The PDI efficacy of hydrogel was examined in vitro against the biofilms of Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa). Confocal scanning laser microscopy (CSLM) was performed to investigate the penetration level of TBO into viable S. aureus biofilms. Incorporation of HMPC could increase the physicochemical properties of chitosan hydrogel including the hardness, viscosity as well as bioadhesion; however, higher HMPC concentration also resulted in reduced antimicrobial effect. CSLM analysis further demonstrated that higher HPMC concentration constrained TBO diffusion into the biofilm. The incubation of biofilm and hydrogel was further performed at an angle of 90 degrees. After light irradiation, compared to the mixture of TBO and chitosan, the hydrogel treated sample showed increased PDI efficacy indicated that incorporation of HPMC did improve antimicrobial effect. Finally, the bactericidal efficacy could be significantly augmented by prolonged retention of hydrogel in the biofilm as well as in the animal model of rat skin burn wounds after light irradiation.
Keywords: chitosan; hydrogel; photodynamic inactivation; toluidine blue O.
Figures


References
-
- Wainwright M., Crossley K. Photosensitizing agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeterior. Biodegrad. 2004;53:119–126. doi: 10.1016/j.ibiod.2003.11.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

