Physical stability of drugs after storage above and below the glass transition temperature: Relationship to glass-forming ability
- PMID: 26341321
- PMCID: PMC4622963
- DOI: 10.1016/j.ijpharm.2015.08.101
Physical stability of drugs after storage above and below the glass transition temperature: Relationship to glass-forming ability
Abstract
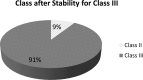
Amorphous materials are inherently unstable and tend to crystallize upon storage. In this study, we investigated the extent to which the physical stability and inherent crystallization tendency of drugs are related to their glass-forming ability (GFA), the glass transition temperature (Tg) and thermodynamic factors. Differential scanning calorimetry was used to produce the amorphous state of 52 drugs [18 compounds crystallized upon heating (Class II) and 34 remained in the amorphous state (Class III)] and to perform in situ storage for the amorphous material for 12h at temperatures 20°C above or below the Tg. A computational model based on the support vector machine (SVM) algorithm was developed to predict the structure-property relationships. All drugs maintained their Class when stored at 20°C below the Tg. Fourteen of the Class II compounds crystallized when stored above the Tg whereas all except one of the Class III compounds remained amorphous. These results were only related to the glass-forming ability and no relationship to e.g. thermodynamic factors was found. The experimental data were used for computational modeling and a classification model was developed that correctly predicted the physical stability above the Tg. The use of a large dataset revealed that molecular features related to aromaticity and π-π interactions reduce the inherent physical stability of amorphous drugs.
Keywords: Amorphous; Computational prediction; Glass-forming ability; Physical stability; SVM.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures






References
-
- Alhalaweh A., Alzghoul A., Kaialy W. Data mining of solubility parameters for computational prediction of drug-excipient miscibility. Drug. Dev. Ind. Pharm. 2013;40:p904–909. - PubMed
-
- Alhalaweh A., Alzghoul A., Waseem K., Denny M., Bergstrom C.A.S. Computational predictions of glass-forming ability and crystallization tendency of drug molecules. Mol. Pharm. 2014;11:3123–3132. - PubMed
-
- Alonso M., Geerlings P., De Proft F. Exploring the structure–aromaticity relationship in Hückel and Möbius N-fused pentaphyrins using DFT. Phys. Chem. Chem. Phys. 2014;16:14396–14407. - PubMed
-
- Alzghoul A., Alhalaweh A., Mahlin D., Bergström C.A. Experimental and computational prediction of glass transition temperature of drugs. J. Chem. Inf. Model. 2014;54:3396–3403. - PubMed
-
- Andronis V., Zografi G. The molecular mobility of supercooled amorphous indomethacin as a function of temperature and relative humidity. Pharm. Res. 1998;15:p835–842. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

