Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage
- PMID: 26341399
- PMCID: PMC4582788
- DOI: 10.1016/j.immuni.2015.08.008
Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage
Abstract
Endoplasmic reticulum (ER) stress is observed in many human diseases, often associated with inflammation. ER stress can trigger inflammation through nucleotide-binding domain and leucine-rich repeat containing (NLRP3) inflammasome, which might stimulate inflammasome formation by association with damaged mitochondria. How ER stress triggers mitochondrial dysfunction and inflammasome activation is ill defined. Here we have used an infection model to show that the IRE1α ER stress sensor regulates regulated mitochondrial dysfunction through an NLRP3-mediated feed-forward loop, independently of ASC. IRE1α activation increased mitochondrial reactive oxygen species, promoting NLRP3 association with mitochondria. NLRP3 was required for ER stress-induced cleavage of caspase-2 and the pro-apoptotic factor, Bid, leading to subsequent release of mitochondrial contents. Caspase-2 and Bid were necessary for activation of the canonical inflammasome by infection-associated or general ER stress. These data identify an NLRP3-caspase-2-dependent mechanism that relays ER stress to the mitochondria to promote inflammation, integrating cellular stress and innate immunity.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
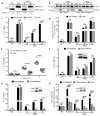


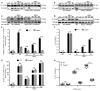


Comment in
-
Stressed-Out Endoplasmic Reticulum Inflames the Mitochondria.Immunity. 2015 Sep 15;43(3):409-11. doi: 10.1016/j.immuni.2015.08.027. Immunity. 2015. PMID: 26377891
References
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI063331/AI/NIAID NIH HHS/United States
- GM007544/GM/NIGMS NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- T32 HL007517/HL/NHLBI NIH HHS/United States
- R37 AI063331/AI/NIAID NIH HHS/United States
- AR059688/AR/NIAMS NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- AA021751/AA/NIAAA NIH HHS/United States
- R01 AR059688/AR/NIAMS NIH HHS/United States
- R01 AA021751/AA/NIAAA NIH HHS/United States
- HL007517/HL/NHLBI NIH HHS/United States
- R21 AI101777/AI/NIAID NIH HHS/United States
- AI063331/AI/NIAID NIH HHS/United States
- R01 AI083713/AI/NIAID NIH HHS/United States
- AI101777/AI/NIAID NIH HHS/United States
- AI083713/AI/NIAID NIH HHS/United States
- R01 AI063331/AI/NIAID NIH HHS/United States
- T32 GM007544/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

