Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program
- PMID: 26341685
- PMCID: PMC4833390
- DOI: 10.1016/j.drugpo.2015.08.008
Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program
Abstract
Background: People who inject drugs (PWID) constitute 10 million people globally with hepatitis C virus, including many opioid agonist treatment patients. Little data exist describing clinical outcomes for patients receiving HCV treatment with direct-acting antiviral agents (DAAs) in opioid agonist treatment settings.
Methods: In this retrospective observational study, we describe clinical outcomes for 50 genotype-1 patients receiving HCV treatment with triple therapy: telaprevir (n=42) or boceprevir (n=8) in combination with pegylated interferon and ribavirin on-site in an opioid agonist treatment program.
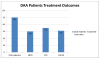
Results: Overall, 70% achieved an end of treatment response (ETR) and 62% achieved a sustained virological response (SVR). These treatment outcomes are nearly equivalent to previously published HCV outcomes shown in registration trials, despite high percentages of recent drug use prior to treatment (52%), ongoing drug use during treatment (45%) and psychiatric comorbidity (86%). Only 12% (n=6) discontinued antiviral treatment early for non-virological reasons. Four patients received a blood transfusion, and one discontinued telaprevir due to severe rash.
Conclusions: These data demonstrate that on-site HCV treatment with direct-acting antiviral agents is effective in opioid agonist treatment patients including patients who are actively using drugs. Future interferon-free regimens will likely be even more effective. Opioid agonist treatment programs represent an opportunity to safely and effectively treat chronic hepatitis C, and PWID should have unrestricted access to DAAs.
Keywords: Direct-acting antiviral agents; HCV; IDU; Injection drug users; PWID; PWUD.
Copyright © 2015 Elsevier B.V. All rights reserved.
References
-
- Alavi M, Raffa JD, Deans GD, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver International. 2014;34(8):1198–1206. - PubMed
-
- Aspinall E, Corson S, Doyle J, Grebely J, Hutchinson SJ, Dore GJ, et al. Peginterferon and ribavirin treatment for chronic hepatitis C in people who inject drugs: a systematic review and meta-analysis. Clin Infect Dis 2012 - PubMed
-
- Barua S, Greenwald R, Yekta S, Swan T, Taylor L. Sofosbuvir and Medicaid: getting it right. JAMA. 2015
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources