Stimuli-responsive nanoparticles for targeting the tumor microenvironment
- PMID: 26341694
- PMCID: PMC4656063
- DOI: 10.1016/j.jconrel.2015.08.050
Stimuli-responsive nanoparticles for targeting the tumor microenvironment
Abstract
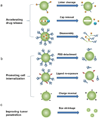
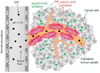
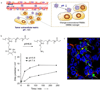
One of the most challenging and clinically important goals in nanomedicine is to deliver imaging and therapeutic agents to solid tumors. Here we discuss the recent design and development of stimuli-responsive smart nanoparticles for targeting the common attributes of solid tumors such as their acidic and hypoxic microenvironments. This class of stimuli-responsive nanoparticles is inactive during blood circulation and under normal physiological conditions, but is activated by acidic pH, enzymatic up-regulation, or hypoxia once they extravasate into the tumor microenvironment. The nanoparticles are often designed to first "navigate" the body's vascular system, "dock" at the tumor sites, and then "activate" for action inside the tumor interstitial space. They combine the favorable biodistribution and pharmacokinetic properties of nanodelivery vehicles and the rapid diffusion and penetration properties of smaller drug cargos. By targeting the broad tumor habitats rather than tumor-specific receptors, this strategy has the potential to overcome the tumor heterogeneity problem and could be used to design diagnostic and therapeutic nanoparticles for a broad range of solid tumors.
Keywords: Hypoxia; Matrix metalloproteinases; Nanomedicine; Tumor heterogeneity; Tumor microenvironment; pH.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
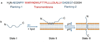


References
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. - PubMed
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161–171. - PubMed
-
- Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng. 2007;9:257–288. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

