Prosaposin activates the androgen receptor and potentiates resistance to endocrine treatment in breast cancer
- PMID: 26341737
- PMCID: PMC4560928
- DOI: 10.1186/s13058-015-0636-6
Prosaposin activates the androgen receptor and potentiates resistance to endocrine treatment in breast cancer
Abstract
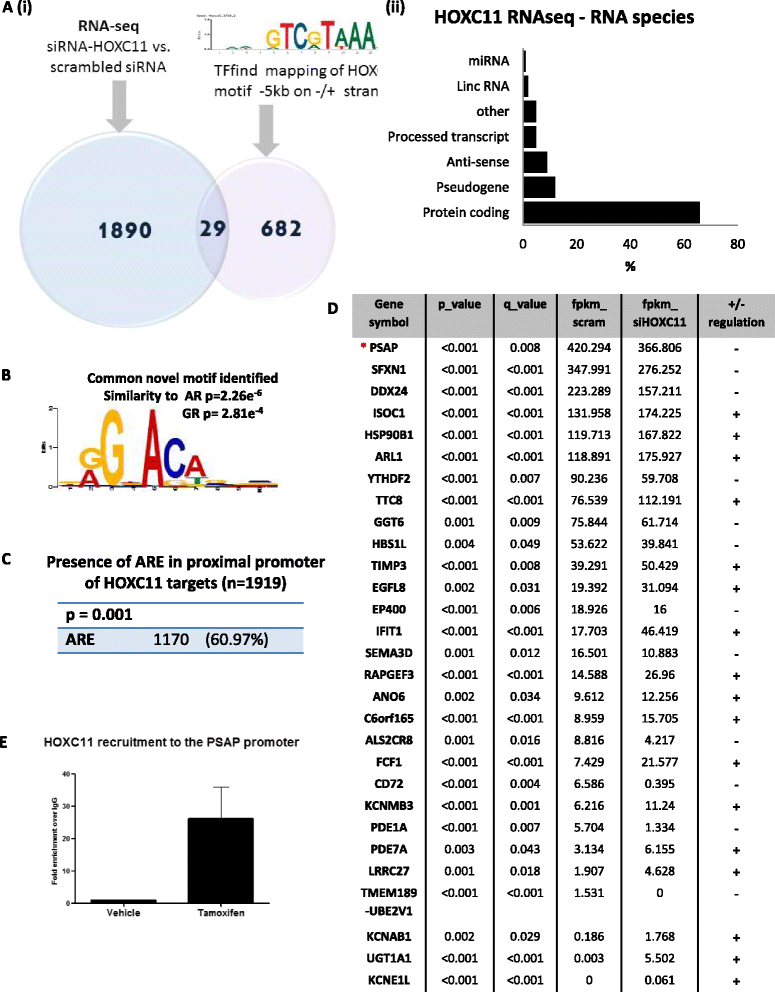
Introduction: HOX genes play vital roles in growth and development, however, atypical redeployment of these genes is often associated with steroidal adaptability in endocrine cancers. We previously identified HOXC11 to be an indicator of poor response to hormonal therapy in breast cancer. In this study we aimed to elucidate genes regulated by HOXC11 in the endocrine resistant setting.
Methods: RNA-sequencing paired with transcription factor motif-mapping was utilised to identify putative HOXC11 target genes in endocrine resistant breast cancer. Validation and functional evaluation of the target gene, prosaposin (PSAP), was performed in a panel of endocrine sensitive and resistant breast cancer cell lines. The clinical significance of this finding was explored in clinical cohorts at both mRNA and protein level.
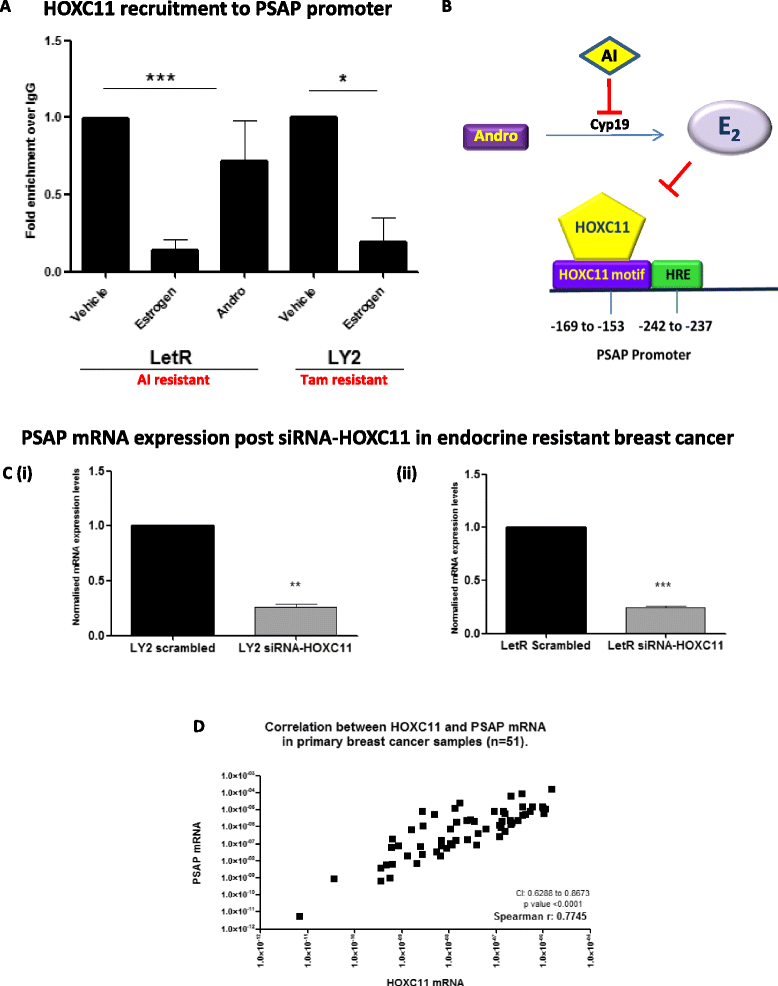
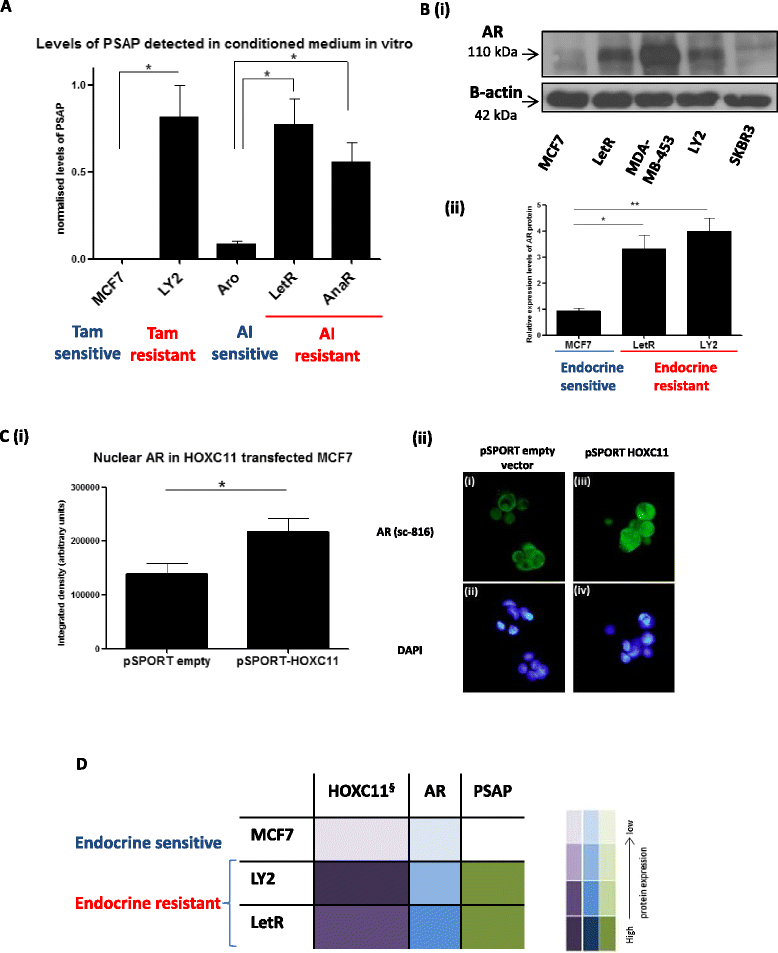
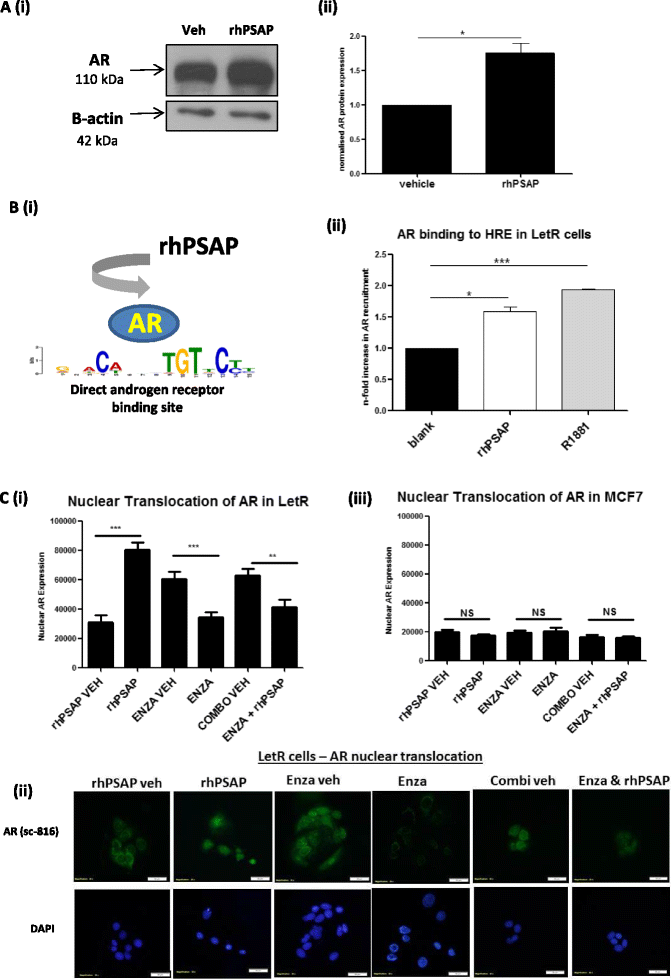
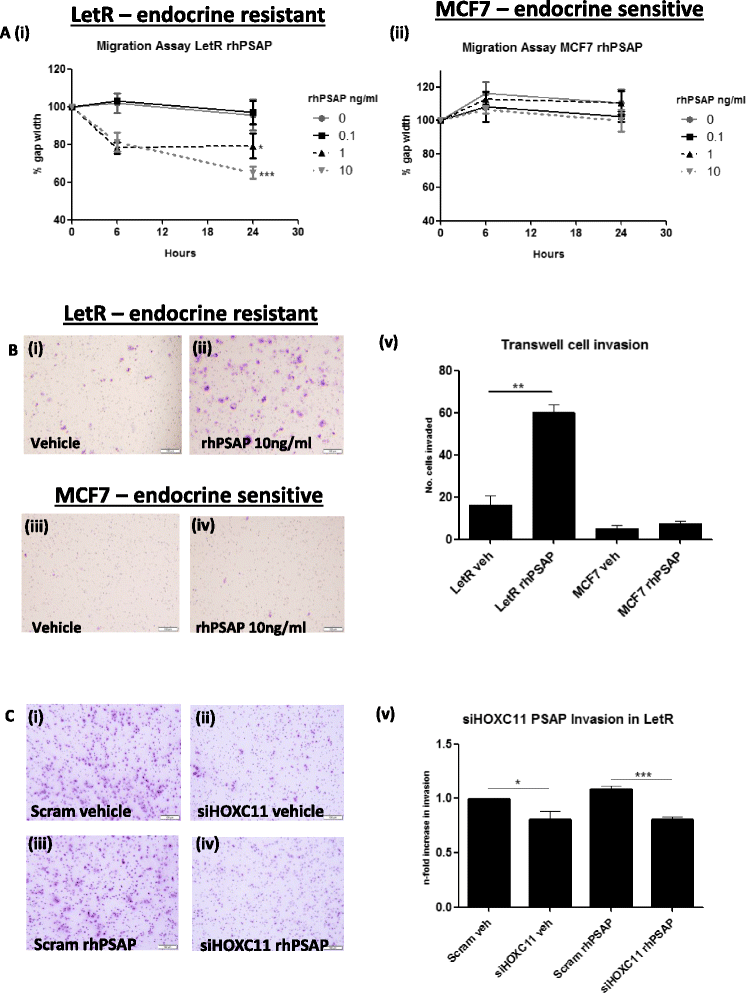
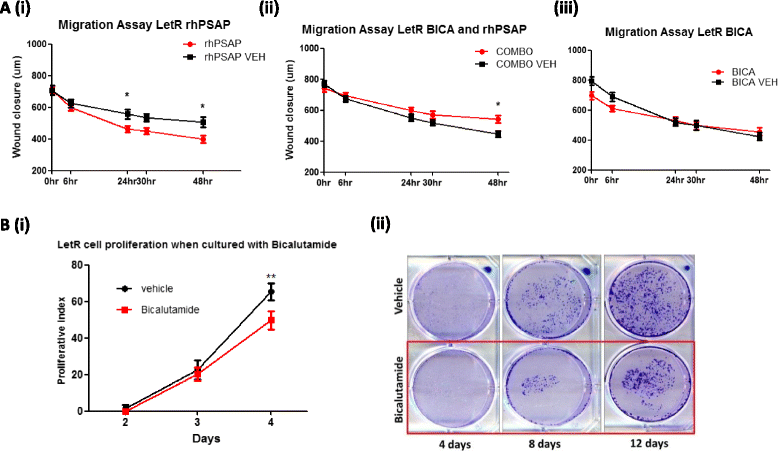
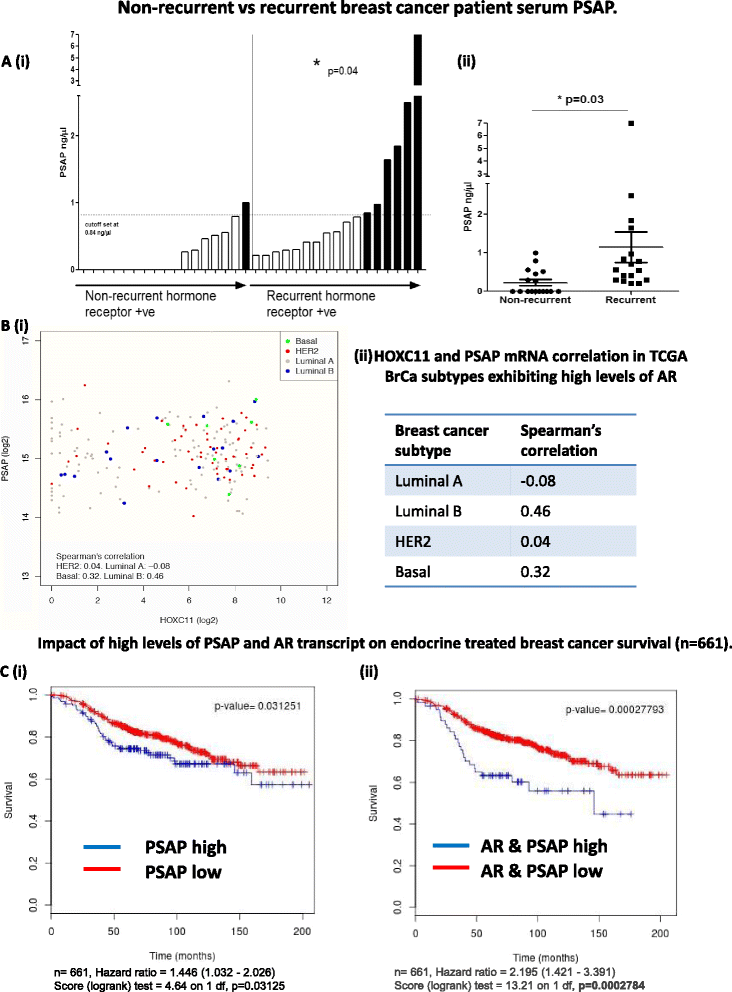
Results: PSAP was shown to be regulated by HOXC11 in both tamoxifen and aromatase inhibitor (AI) resistant cell lines. Transcript levels of HOXC11 and PSAP correlated strongly in samples of primary breast tumours (r = 0.7692, n = 51). PSAP has previously been reported to activate androgen receptor (AR) in prostate cancer cells. In a panel of breast cancer cell lines it was shown that endocrine resistant cells exhibit innately elevated levels of AR compared to their endocrine sensitive counterparts. Here, we demonstrate that stimulation with PSAP can drive AR recruitment to a hormone response element (HRE) in AI resistant breast cancer cells. Functionally, PSAP promotes cell migration and invasion only in AI resistant cells and not in their endocrine sensitive counterparts. In a cohort of breast cancer patients (n = 34), elevated serum levels of PSAP were found to associate significantly with poor response to endocrine treatment (p = 0.04). Meta-analysis of combined PSAP and AR mRNA are indicative of poor disease-free survival in endocrine treated breast cancer patients (hazard ratio (HR): 2.2, P = 0.0003, n = 661).
Conclusion: The HOXC11 target gene, PSAP, is an AR activator which facilitates adaptation to a more invasive phenotype in vitro. These findings have particular relevance to the development of resistance to AI therapy which is an emerging clinical issue. PSAP is a secreted biomarker which has potential in identifying patients failing to exhibit sustained response to hormonal treatment.
Figures
Similar articles
-
TSC22D1 and PSAP predict clinical outcome of tamoxifen treatment in patients with recurrent breast cancer.Breast Cancer Res Treat. 2009 Jan;113(2):253-60. doi: 10.1007/s10549-008-9934-3. Epub 2008 Feb 26. Breast Cancer Res Treat. 2009. PMID: 18299979
-
Prosaposin is an AR-target gene and its neurotrophic domain upregulates AR expression and activity in prostate stromal cells.J Cell Biochem. 2008 Aug 15;104(6):2272-85. doi: 10.1002/jcb.21786. J Cell Biochem. 2008. PMID: 18481277
-
MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer.Breast Cancer Res. 2015 Jan 30;17(1):13. doi: 10.1186/s13058-015-0515-1. Breast Cancer Res. 2015. PMID: 25633049 Free PMC article.
-
The development, application and limitations of breast cancer cell lines to study tamoxifen and aromatase inhibitor resistance.J Steroid Biochem Mol Biol. 2012 Sep;131(3-5):83-92. doi: 10.1016/j.jsbmb.2011.12.005. Epub 2012 Jan 8. J Steroid Biochem Mol Biol. 2012. PMID: 22265958 Free PMC article. Review.
-
Endocrine therapy and other targeted therapies for metastatic breast cancer.Expert Rev Anticancer Ther. 2004 Dec;4(6):1179-95. doi: 10.1586/14737140.4.6.1179. Expert Rev Anticancer Ther. 2004. PMID: 15606341 Review.
Cited by
-
HOXC11 drives lung adenocarcinoma progression through transcriptional regulation of SPHK1.Cell Death Dis. 2023 Feb 23;14(2):153. doi: 10.1038/s41419-023-05673-8. Cell Death Dis. 2023. PMID: 36823149 Free PMC article.
-
Research advances of secretory proteins in malignant tumors.Chin J Cancer Res. 2021 Feb 28;33(1):115-132. doi: 10.21147/j.issn.1000-9604.2021.01.12. Chin J Cancer Res. 2021. PMID: 33707934 Free PMC article.
-
N-Glycoproteomics Study of Putative N-Glycoprotein Biomarkers of Drug Resistance in MCF-7/ADR Cells.Phenomics. 2021 Oct 28;1(6):269-284. doi: 10.1007/s43657-021-00029-8. eCollection 2021 Dec. Phenomics. 2021. PMID: 36939756 Free PMC article.
-
Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation.FASEB J. 2019 Aug;33(8):9263-9278. doi: 10.1096/fj.201801483RR. Epub 2019 May 21. FASEB J. 2019. PMID: 31112400 Free PMC article.
-
A nuclear-directed human pancreatic ribonuclease (PE5) targets the metabolic phenotype of cancer cells.Oncotarget. 2016 Apr 5;7(14):18309-24. doi: 10.18632/oncotarget.7579. Oncotarget. 2016. PMID: 26918450 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

