Frequency of RANTES gene polymorphisms and their association with incidence of malaria: a longitudinal study on children in Iganga district, Uganda
- PMID: 26341782
- PMCID: PMC4560921
- DOI: 10.1186/s12936-015-0875-0
Frequency of RANTES gene polymorphisms and their association with incidence of malaria: a longitudinal study on children in Iganga district, Uganda
Abstract
Background: The severity and outcome of malaria is influenced by host immunity in which chemokines such as Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES) play an important role. Previous studies show that variations in the RANTES gene affect RANTES protein production, hence altering host immunity. In this study, the relationship between presence of mutations in RANTES and incidence of malaria in a cohort of children living in a malaria-endemic area of Uganda was determined.
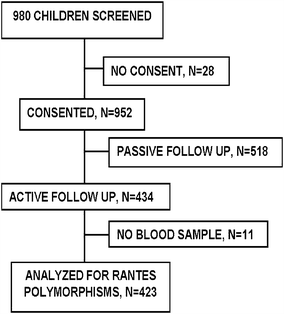
Methods: This was a longitudinal study comprising of 423 children aged between 6 months and 9 years, who were actively followed up for 1 year. Malaria episodes occurring in the cohort children were detected and the affected children treated with national policy drug regimen. Mutations in the RANTES gene were determined by PCR-RFLP method and their frequencies were calculated. A multivariate negative binomial regression model was used to estimate the impact of RANTES mutations on malaria incidence. In all statistical tests, a P-value of <0.05 was considered as significant.
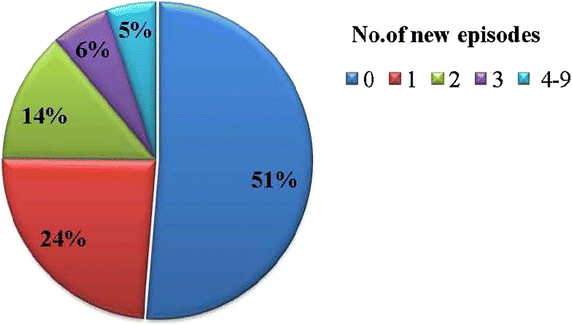
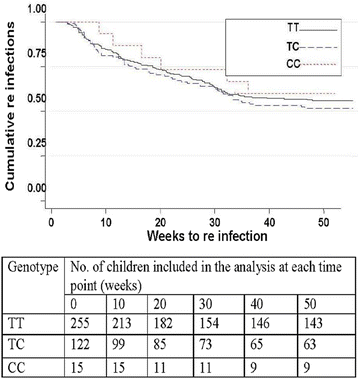
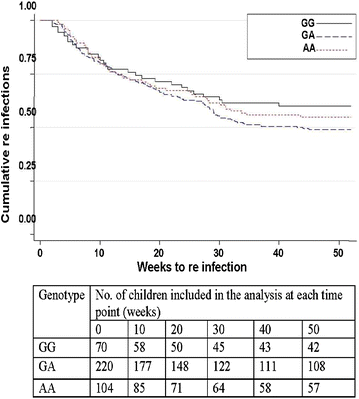
Results: The frequencies of the -403A and In1.1C allele were 53.7 and 19.2 %, respectively. No mutations were found at the -28 locus. After adjustment of incidence rates for age, blood group, insecticide-treated bed net (ITN) use, malaria history and the sickle cell trait, 1n1.1T/C heterozygotes and homozygotes showed a non-significant trend towards higher incidence rates compared to wild-type individuals (IRR = 1.10; P = 0.55 and IRR = 1.25; P = 0.60, respectively). Similarly, there was no significant difference in malaria incidence rates between RANTES -403G/A heterozygotes or homozygotes and those without mutations (IRR = 1.09; P = 0.66 and IRR = 1.16; P = 0.50, respectively). No relation was seen between RANTES polymorphisms, baseline parasite densities and the time to first re-infection after administration of anti-malaria drugs.
Conclusions: This study showed that the -403A mutation occurs in nearly half of the study population and the In1.1C allele occurs in one in every four children. Despite the high frequency of these mutations, there was no clear association with malaria incidence. Other studies evaluating more markers, that could potentially modulate RANTES gene transcription alongside other genetic modifiers of malaria susceptibility, may provide further explanations to these less dramatic findings.
Figures
Similar articles
-
Haptoglobin gene diversity and incidence of uncomplicated malaria among children in Iganga, Uganda.Malar J. 2020 Nov 26;19(1):435. doi: 10.1186/s12936-020-03515-y. Malar J. 2020. PMID: 33243242 Free PMC article.
-
Prevalence of polymorphisms in glucose-6-phosphate dehydrogenase, sickle haemoglobin and nitric oxide synthase genes and their relationship with incidence of uncomplicated malaria in Iganga, Uganda.Malar J. 2017 Aug 9;16(1):322. doi: 10.1186/s12936-017-1970-1. Malar J. 2017. PMID: 28793894 Free PMC article.
-
[Preliminary study on the association of chemokine RANTES gene polymorphisms with HIV-1 infection in Chinese Han population].Zhonghua Liu Xing Bing Xue Za Zhi. 2003 Nov;24(11):971-5. Zhonghua Liu Xing Bing Xue Za Zhi. 2003. PMID: 14687494 Chinese.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The regulated upon activation normal T-cell expressed and secreted (RANTES) -28C/G and -403G/A polymorphisms and asthma risk: a meta-analysis.Mol Diagn Ther. 2014 Oct;18(5):523-31. doi: 10.1007/s40291-014-0112-5. Mol Diagn Ther. 2014. PMID: 25004906 Review.
Cited by
-
Effect of ABO blood group on asymptomatic, uncomplicated and placental Plasmodium falciparum infection: systematic review and meta-analysis.BMC Infect Dis. 2019 Jan 25;19(1):86. doi: 10.1186/s12879-019-3730-z. BMC Infect Dis. 2019. PMID: 30683058 Free PMC article.
-
Haptoglobin gene diversity and incidence of uncomplicated malaria among children in Iganga, Uganda.Malar J. 2020 Nov 26;19(1):435. doi: 10.1186/s12936-020-03515-y. Malar J. 2020. PMID: 33243242 Free PMC article.
-
Prevalence of polymorphisms in glucose-6-phosphate dehydrogenase, sickle haemoglobin and nitric oxide synthase genes and their relationship with incidence of uncomplicated malaria in Iganga, Uganda.Malar J. 2017 Aug 9;16(1):322. doi: 10.1186/s12936-017-1970-1. Malar J. 2017. PMID: 28793894 Free PMC article.
-
Association between RANTES/CCL5 levels with Plasmodium infections and malaria severity: a systematic review.Malar J. 2024 Nov 9;23(1):335. doi: 10.1186/s12936-024-05152-1. Malar J. 2024. PMID: 39521981 Free PMC article.
-
Genetic polymorphisms as multi-biomarkers in severe acute respiratory syndrome (SARS) by coronavirus infection: A systematic review of candidate gene association studies.Infect Genet Evol. 2021 Sep;93:104846. doi: 10.1016/j.meegid.2021.104846. Epub 2021 Apr 30. Infect Genet Evol. 2021. PMID: 33933633 Free PMC article.
References
-
- WHO . World malaria report 2014. Geneva: World Health Organization; 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

