Developmental expression and function analysis of protein tyrosine phosphatase receptor type D in oligodendrocyte myelination
- PMID: 26341907
- PMCID: PMC4600676
- DOI: 10.1016/j.neuroscience.2015.08.062
Developmental expression and function analysis of protein tyrosine phosphatase receptor type D in oligodendrocyte myelination
Abstract
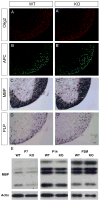
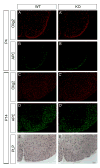
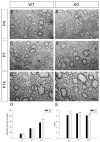
Receptor protein tyrosine phosphatases (RPTPs) are extensively expressed in the central nervous system (CNS), and have distinct spatial and temporal patterns in different cell types during development. Previous studies have demonstrated possible roles for RPTPs in axon outgrowth, guidance, and synaptogenesis. In the present study, our results revealed that protein tyrosine phosphatase, receptor type D (PTPRD) was initially expressed in mature neurons in embryonic CNS, and later in oligodendroglial cells at postnatal stages when oligodendrocytes undergo active axonal myelination process. In PTPRD mutants, oligodendrocyte differentiation was normal and a transient myelination delay occurred at early postnatal stages, indicating the contribution of PTPRD to the initiation of axonal myelination. Our results also showed that the remyelination process was not affected in the absence of PTPRD function after a cuprizone-induced demyelination in adult animals.
Keywords: axonal myelination; corpus callosum; cuprizone; spinal cord.
Published by Elsevier Ltd.
Figures


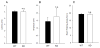


Similar articles
-
Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination.J Neurosci. 2018 Feb 14;38(7):1802-1820. doi: 10.1523/JNEUROSCI.1291-17.2018. Epub 2018 Jan 15. J Neurosci. 2018. PMID: 29335358 Free PMC article.
-
Inactivation of Protein Tyrosine Phosphatase Receptor Type Z by Pleiotrophin Promotes Remyelination through Activation of Differentiation of Oligodendrocyte Precursor Cells.J Neurosci. 2015 Sep 2;35(35):12162-71. doi: 10.1523/JNEUROSCI.2127-15.2015. J Neurosci. 2015. PMID: 26338327 Free PMC article.
-
Apoptosis of Oligodendrocytes during Early Development Delays Myelination and Impairs Subsequent Responses to Demyelination.J Neurosci. 2015 Oct 14;35(41):14031-41. doi: 10.1523/JNEUROSCI.1706-15.2015. J Neurosci. 2015. PMID: 26468203 Free PMC article.
-
rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination.Neurobiol Dis. 2017 Sep;105:142-155. doi: 10.1016/j.nbd.2017.05.015. Epub 2017 May 30. Neurobiol Dis. 2017. PMID: 28576706
-
Heterogeneity in oligodendroglia: Is it relevant to mouse models and human disease?J Neurosci Res. 2016 Dec;94(12):1421-1433. doi: 10.1002/jnr.23900. Epub 2016 Aug 25. J Neurosci Res. 2016. PMID: 27557736 Free PMC article. Review.
Cited by
-
Alternatively spliced mini-exon B in PTPδ regulates excitatory synapses through cell-type-specific trans-synaptic PTPδ-IL1RAP interaction.Nat Commun. 2025 May 13;16(1):4415. doi: 10.1038/s41467-025-59685-3. Nat Commun. 2025. PMID: 40360498 Free PMC article.
-
Effects of Multiple Genetic Loci on Age at Onset in Frontotemporal Dementia.J Alzheimers Dis. 2017;56(4):1271-1278. doi: 10.3233/JAD-160949. J Alzheimers Dis. 2017. PMID: 28128768 Free PMC article.
-
Single-nucleus transcriptomic profiling of multiple organs in a rhesus macaque model of SARS-CoV-2 infection.Zool Res. 2022 Nov 18;43(6):1041-1062. doi: 10.24272/j.issn.2095-8137.2022.443. Zool Res. 2022. PMID: 36349357 Free PMC article.
-
Neural conditional ablation of the protein tyrosine phosphatase receptor Delta PTPRD impairs gliogenesis in the developing mouse brain cortex.Front Cell Dev Biol. 2024 Feb 29;12:1357862. doi: 10.3389/fcell.2024.1357862. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38487272 Free PMC article.
-
PTPRD and CNTNAP2 as markers of tumor aggressiveness in oligodendrogliomas.Sci Rep. 2022 Aug 18;12(1):14083. doi: 10.1038/s41598-022-14977-2. Sci Rep. 2022. PMID: 35982066 Free PMC article.
References
-
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. - PubMed
-
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. - PubMed
-
- Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

