ESCRT-III-Associated Protein ALIX Mediates High-Affinity Phosphate Transporter Trafficking to Maintain Phosphate Homeostasis in Arabidopsis
- PMID: 26342016
- PMCID: PMC4815105
- DOI: 10.1105/tpc.15.00393
ESCRT-III-Associated Protein ALIX Mediates High-Affinity Phosphate Transporter Trafficking to Maintain Phosphate Homeostasis in Arabidopsis
Erratum in
-
Correction to: ESCRT-III-Associated Protein ALIX Mediates High-Affinity Phosphate Transporter Trafficking to Maintain Phosphate Homeostasis in Arabidopsis.Plant Cell. 2022 Jul 4;34(7):2809. doi: 10.1093/plcell/koac103. Plant Cell. 2022. PMID: 35348792 Free PMC article. No abstract available.
Abstract
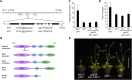
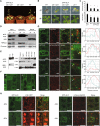
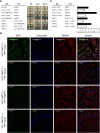
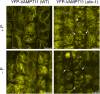
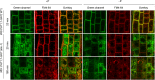
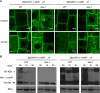
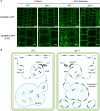
Prior to the release of their cargoes into the vacuolar lumen, sorting endosomes mature into multivesicular bodies (MVBs) through the action of ENDOSOMAL COMPLEX REQUIRED FOR TRANSPORT (ESCRT) protein complexes. MVB-mediated sorting of high-affinity phosphate transporters (PHT1) to the vacuole limits their plasma membrane levels under phosphate-sufficient conditions, a process that allows plants to maintain phosphate homeostasis. Here, we describe ALIX, a cytosolic protein that associates with MVB by interacting with ESCRT-III subunit SNF7 and mediates PHT1;1 trafficking to the vacuole in Arabidopsis thaliana. We show that the partial loss-of-function mutant alix-1 displays reduced vacuolar degradation of PHT1;1. ALIX derivatives containing the alix-1 mutation showed reduced interaction with SNF7, providing a simple molecular explanation for impaired cargo trafficking in alix-1 mutants. In fact, the alix-1 mutation also hampered vacuolar sorting of the brassinosteroid receptor BRI1. We also show that alix-1 displays altered vacuole morphogenesis, implying a new role for ALIX proteins in vacuolar biogenesis, likely acting as part of ESCRT-III complexes. In line with a presumed broad target spectrum, the alix-1 mutation is pleiotropic, leading to reduced plant growth and late flowering, with stronger alix mutations being lethal, indicating that ALIX participates in diverse processes in plants essential for their life.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures



References
-
- Ames B.N. (1966). Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8: 115–118.
-
- Aubert S., Alban C., Bligny R., Douce R. (1996). Induction of beta-methylcrotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: evidence for a role of MCCase in leucine catabolism. FEBS Lett. 383: 175–180. - PubMed
-
- Babst M. (2005). A protein’s final ESCRT. Traffic 6: 2–9. - PubMed
-
- Babst M., Katzmann D.J., Estepa-Sabal E.J., Meerloo T., Emr S.D. (2002a). Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3: 271–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

