MAPRE1 as a plasma biomarker for early-stage colorectal cancer and adenomas
- PMID: 26342024
- PMCID: PMC4633385
- DOI: 10.1158/1940-6207.CAPR-15-0077
MAPRE1 as a plasma biomarker for early-stage colorectal cancer and adenomas
Abstract
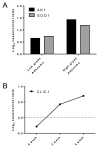
Blood-based biomarkers for early detection of colorectal cancer could complement current approaches to colorectal cancer screening. We previously identified the APC-binding protein MAPRE1 as a potential colorectal cancer biomarker. Here, we undertook a case-control validation study to determine the performance of MAPRE1 in detecting early colorectal cancer and colon adenoma and to assess the potential relevance of additional biomarker candidates. We analyzed plasma samples from 60 patients with adenomas, 30 with early colorectal cancer, 30 with advanced colorectal cancer, and 60 healthy controls. MAPRE1 and a set of 21 proteins with potential biomarker utility were assayed using high-density antibody arrays, and carcinoembryonic antigen (CEA) was assayed using ELISA. The biologic significance of the candidate biomarkers was also assessed in colorectal cancer mouse models. Plasma MAPRE1 levels were significantly elevated in both patients with adenomas and patients with colorectal cancer compared with controls (P < 0.0001). MAPRE1 and CEA together yielded an area under the curve of 0.793 and a sensitivity of 0.400 at 95% specificity for differentiating early colorectal cancer from controls. Three other biomarkers (AK1, CLIC1, and SOD1) were significantly increased in both adenoma and early colorectal cancer patient plasma samples and in plasma from colorectal cancer mouse models at preclinical stages compared with controls. The combination of MAPRE1, CEA, and AK1 yielded sensitivities of 0.483 and 0.533 at 90% specificity and sensitivities of 0.350 and 0.467 at 95% specificity for differentiating adenoma and early colorectal cancer, respectively, from healthy controls. These findings suggest that MAPRE1 can contribute to the detection of early-stage colorectal cancer and adenomas together with other biomarkers.
©2015 American Association for Cancer Research.
Figures


References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:104–17. - PubMed
-
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. - PubMed
-
- Valori R, Rey JF, Atkin WS, Bretthauer M, Senore C, Hoff G, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012;44(Suppl 3):SE88–105. - PubMed
-
- Kuipers EJ, Rosch T, Bretthauer M. Colorectal cancer screening--optimizing current strategies and new directions. Nature reviews Clinical oncology. 2013;10:130–42. - PubMed
-
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. The New England journal of medicine. 2014;370:1287–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

