Binding of Galectin-3, a β-Galactoside-binding Lectin, to MUC1 Protein Enhances Phosphorylation of Extracellular Signal-regulated Kinase 1/2 (ERK1/2) and Akt, Promoting Tumor Cell Malignancy
- PMID: 26342075
- PMCID: PMC4646264
- DOI: 10.1074/jbc.M115.651489
Binding of Galectin-3, a β-Galactoside-binding Lectin, to MUC1 Protein Enhances Phosphorylation of Extracellular Signal-regulated Kinase 1/2 (ERK1/2) and Akt, Promoting Tumor Cell Malignancy
Abstract
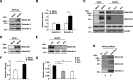
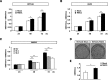
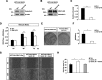
Both mucin 1 (MUC1) and galectin-3 are known to be overexpressed in various malignant tumors and associated with a poor prognosis. It has been extensively reported that MUC1 is involved in potentiation of growth factor-dependent signal transduction. Because some carbohydrate moieties carried on MUC1 change to preferable ones for binding of galectin-3 in cancer cells, we speculated that MUC1-mediated signaling may occur through direct binding of galectin-3. Immunochemical studies showed that the distribution of galectin-3 coincided with that of MUC1 in various human tumor tissues but not in human nonmalignant tissues, and the level of galectin-3 retained on the surface of various cancer cells paralleled that of MUC1. Treatment of MUC1-expressing cells with galectin-3 induced phosphorylation of ERK1/2 and Akt following enhanced phosphorylation of MUC1 C-terminal domain, consistently promoting tumor cell malignancy. It is also noted that this enhanced phosphorylation occurred independently of EGF receptor-mediated signaling in both EGF receptor- and MUC1-expressing cells, and multivalency of galectin-3 was important for initiation of MUC1-mediated signaling. Expectedly, both silencing of endogenous galectin-3 and treatment with galectin-3 antagonists down-regulated cell proliferation of MUC1-expressing cells. These results suggest that the binding of galectin-3 to MUC1 plays a key role in MUC1-mediated signaling. Thus, constitutive activation of MUC1-mediated signaling in an autocrine/paracrine manner caused by ligation of galectin-3 promotes uncontrolled tumor cell malignancy. This signaling may be another MUC1-mediated pathway and function in parallel with a growth factor-dependent MUC1-mediated signaling pathway.
Keywords: cancer; cell motility; cell proliferation; galectin-3; mucin 1, cell surface-associated (MUC1); signal transduction.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








References
-
- Kufe D., Inghirami G., Abe M., Hayes D., Justi-Wheeler H., and Schlom J. (1984) Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma 3, 223–232 - PubMed
-
- Ligtenberg M. J., Kruijshaar L., Buijs F., van Meijer M., Litvinov S. V., and Hilkens J. (1992) Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 267, 6171–6177 - PubMed
-
- Parry S., Silverman H. S., McDermott K., Willis A., Hollingsworth M. A., and Harris A. (2001) Identification of MUC1 proteolytic cleavage sites in vivo. Biochem. Biophys. Res. Commun. 283, 715–720 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

