Mapping Key Residues of ISD11 Critical for NFS1-ISD11 Subcomplex Stability: IMPLICATIONS IN THE DEVELOPMENT OF MITOCHONDRIAL DISORDER, COXPD19
- PMID: 26342079
- PMCID: PMC4646244
- DOI: 10.1074/jbc.M115.678508
Mapping Key Residues of ISD11 Critical for NFS1-ISD11 Subcomplex Stability: IMPLICATIONS IN THE DEVELOPMENT OF MITOCHONDRIAL DISORDER, COXPD19
Abstract
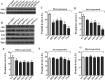
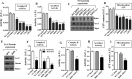
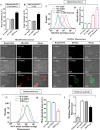
Biogenesis of the iron-sulfur (Fe-S) cluster is an indispensable process in living cells. In mammalian mitochondria, the initial step of the Fe-S cluster assembly process is assisted by the NFS1-ISD11 complex, which delivers sulfur to scaffold protein ISCU during Fe-S cluster synthesis. Although ISD11 is an essential protein, its cellular role in Fe-S cluster biogenesis is still not defined. Our study maps the important ISD11 amino acid residues belonging to putative helix 1 (Phe-40), helix 3 (Leu-63, Arg-68, Gln-69, Ile-72, Tyr-76), and C-terminal segment (Leu-81, Glu-84) are critical for in vivo Fe-S cluster biogenesis. Importantly, mutation of these conserved ISD11 residues into alanine leads to its compromised interaction with NFS1, resulting in reduced stability and enhanced aggregation of NFS1 in the mitochondria. Due to altered interaction with ISD11 mutants, the levels of NFS1 and Isu1 were significantly depleted, which affects Fe-S cluster biosynthesis, leading to reduced electron transport chain complex (ETC) activity and mitochondrial respiration. In humans, a clinically relevant ISD11 mutation (R68L) has been associated in the development of a mitochondrial genetic disorder, COXPD19. Our findings highlight that the ISD11 R68A/R68L mutation display reduced affinity to form a stable subcomplex with NFS1, and thereby fails to prevent NFS1 aggregation resulting in impairment of the Fe-S cluster biogenesis. The prime affected machinery is the ETC complex, which showed compromised redox properties, causing diminished mitochondrial respiration. Furthermore, the R68L ISD11 mutant displayed accumulation of mitochondrial iron and reactive oxygen species, leading to mitochondrial dysfunction, which correlates with the phenotype observed in COXPD19 patients.
Keywords: electron transfer; iron-sulfur protein; mitochondria; mitochondrial disease; molecular chaperone; protein-protein interaction; reactive oxygen species (ROS); respiration.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
The N-Terminus of Iron-Sulfur Cluster Assembly Factor ISD11 Is Crucial for Subcellular Targeting and Interaction with l-Cysteine Desulfurase NFS1.Biochemistry. 2017 Mar 28;56(12):1797-1808. doi: 10.1021/acs.biochem.6b01239. Epub 2017 Mar 17. Biochemistry. 2017. PMID: 28271877
-
Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron-sulfur cluster scaffold protein (ISCU).J Biol Chem. 2013 Oct 4;288(40):28755-70. doi: 10.1074/jbc.M113.482042. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940031 Free PMC article.
-
Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex.Nat Commun. 2017 Nov 3;8(1):1287. doi: 10.1038/s41467-017-01497-1. Nat Commun. 2017. PMID: 29097656 Free PMC article.
-
Mammalian iron-sulfur cluster biogenesis: Recent insights into the roles of frataxin, acyl carrier protein and ATPase-mediated transfer to recipient proteins.Curr Opin Chem Biol. 2020 Apr;55:34-44. doi: 10.1016/j.cbpa.2019.11.014. Epub 2020 Jan 6. Curr Opin Chem Biol. 2020. PMID: 31918395 Free PMC article. Review.
-
The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism.Biochim Biophys Acta. 2012 Sep;1823(9):1491-508. doi: 10.1016/j.bbamcr.2012.05.009. Epub 2012 May 15. Biochim Biophys Acta. 2012. PMID: 22609301 Review.
Cited by
-
Iron-sulfur cluster ISD11 deficiency (LYRM4 gene) presenting as cardiorespiratory arrest and 3-methylglutaconic aciduria.JIMD Rep. 2019 Jul 24;49(1):11-16. doi: 10.1002/jmd2.12058. eCollection 2019 Sep. JIMD Rep. 2019. PMID: 31497476 Free PMC article.
-
Structural and Functional Characterization of CreFH1, the Frataxin Homolog from Chlamydomonas reinhardtii.Plants (Basel). 2022 Jul 26;11(15):1931. doi: 10.3390/plants11151931. Plants (Basel). 2022. PMID: 35893635 Free PMC article.
-
Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):E5325-E5334. doi: 10.1073/pnas.1702849114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634302 Free PMC article.
-
Iron-sulfur cluster biosynthesis and trafficking - impact on human disease conditions.Metallomics. 2018 Jan 24;10(1):9-29. doi: 10.1039/c7mt00180k. Metallomics. 2018. PMID: 29019354 Free PMC article. Review.
-
Structural and Functional Perspectives on Mitochondrial LYR-Domain Proteins in Plants.Physiol Plant. 2025 Jul-Aug;177(4):e70393. doi: 10.1111/ppl.70393. Physiol Plant. 2025. PMID: 40654039 Free PMC article.
References
-
- Craig E. A., Voisine C., and Schilke B. (1999) Mitochondrial iron metabolism in the yeast Saccharomyces cerevisiae. Biol. Chem. 380, 1167–1173 - PubMed
-
- Meyer J. (2008) Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J. Biol. Inorg. Chem. 13, 157–170 - PubMed
-
- Lill R., Dutkiewicz R., Elsässer H. P., Hausmann A., Netz D. J., Pierik A. J., Stehling O., Urzica E., and Mühlenhoff U. (2006) Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes. Biochim. Biophys. Acta 1763, 652–667 - PubMed
-
- Beinert H., Holm R. H., and Münck E. (1997) Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277, 653–659 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

