Inhibition of Pseudomonas aeruginosa biofilm formation on wound dressings
- PMID: 26342168
- PMCID: PMC4980578
- DOI: 10.1111/wrr.12365
Inhibition of Pseudomonas aeruginosa biofilm formation on wound dressings
Abstract
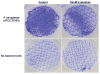
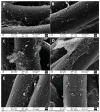
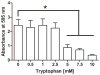
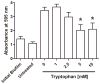
Chronic nonhealing skin wounds often contain bacterial biofilms that prevent normal wound healing and closure and present challenges to the use of conventional wound dressings. We investigated inhibition of Pseudomonas aeruginosa biofilm formation, a common pathogen of chronic skin wounds, on a commercially available biological wound dressing. Building on prior reports, we examined whether the amino acid tryptophan would inhibit P. aeruginosa biofilm formation on the three-dimensional surface of the biological dressing. Bacterial biomass and biofilm polysaccharides were quantified using crystal violet staining or an enzyme linked lectin, respectively. Bacterial cells and biofilm matrix adherent to the wound dressing were visualized through scanning electron microscopy. D-/L-tryptophan inhibited P. aeruginosa biofilm formation on the wound dressing in a dose dependent manner and was not directly cytotoxic to immortalized human keratinocytes although there was some reduction in cellular metabolism or enzymatic activity. More importantly, D-/L-tryptophan did not impair wound healing in a splinted skin wound murine model. Furthermore, wound closure was improved when D-/L-tryptophan treated wound dressing with P. aeruginosa biofilms were compared with untreated dressings. These findings indicate that tryptophan may prove useful for integration into wound dressings to inhibit biofilm formation and promote wound healing.
© 2015 by the Wound Healing Society.
Figures









Similar articles
-
Impact of a novel, antimicrobial dressing on in vivo, Pseudomonas aeruginosa wound biofilm: quantitative comparative analysis using a rabbit ear model.Wound Repair Regen. 2014 Nov-Dec;22(6):712-9. doi: 10.1111/wrr.12232. Epub 2015 Jan 8. Wound Repair Regen. 2014. PMID: 25230854
-
Novel murine model for delayed wound healing using a biological wound dressing with Pseudomonas aeruginosa biofilms.Microb Pathog. 2018 Sep;122:30-38. doi: 10.1016/j.micpath.2018.05.043. Epub 2018 May 26. Microb Pathog. 2018. PMID: 29842898
-
Chronic Pseudomonas aeruginosa biofilm infection impairs murine S100A8/A9 and neutrophil effector cytokines-implications for delayed wound closure?Pathog Dis. 2017 Sep 29;75(7). doi: 10.1093/femspd/ftx068. Pathog Dis. 2017. PMID: 28645160
-
Murine burn lesion model for studying acute and chronic wound infections.APMIS. 2022 Jul;130(7):477-490. doi: 10.1111/apm.13228. Epub 2022 May 16. APMIS. 2022. PMID: 35441434 Review.
-
A review of the scientific evidence for biofilms in wounds.Wound Repair Regen. 2012 Sep-Oct;20(5):647-57. doi: 10.1111/j.1524-475X.2012.00836.x. Wound Repair Regen. 2012. PMID: 22985037 Review.
Cited by
-
Enhancement of Inhibition of the Pseudomonas sp. Biofilm Formation on Bacterial Cellulose-Based Wound Dressing by the Combined Action of Alginate Lyase and Gentamicin.Int J Mol Sci. 2023 Mar 1;24(5):4740. doi: 10.3390/ijms24054740. Int J Mol Sci. 2023. PMID: 36902169 Free PMC article.
-
Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings.Materials (Basel). 2016 May 6;9(5):345. doi: 10.3390/ma9050345. Materials (Basel). 2016. PMID: 28773468 Free PMC article.
-
Repair Process Impairment by Pseudomonas aeruginosa in Epithelial Tissues: Major Features and Potential Therapeutic Avenues.Front Cell Infect Microbiol. 2019 May 31;9:182. doi: 10.3389/fcimb.2019.00182. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31214514 Free PMC article. Review.
-
Anti-biofilm effects of anthranilate on a broad range of bacteria.Sci Rep. 2017 Aug 17;7(1):8604. doi: 10.1038/s41598-017-06540-1. Sci Rep. 2017. PMID: 28819217 Free PMC article.
-
Antibiotic Nanoparticles-Loaded Wound Dressings Against Pseudomonas aeruginosa's Skin Infection: A Systematic Review.Int J Nanomedicine. 2024 Aug 2;19:7895-7926. doi: 10.2147/IJN.S469724. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39108405 Free PMC article.
References
-
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. Epub 2007/12/19. - DOI - PubMed
-
- Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. Journal of bacteriology. 2006;188:8213–21. doi: 10.1128/JB.01202-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

