Interventions for treating constipation in pregnancy
- PMID: 26342714
- PMCID: PMC8958874
- DOI: 10.1002/14651858.CD011448.pub2
Interventions for treating constipation in pregnancy
Abstract
Background: Constipation is a common symptom experienced during pregnancy. It has a range of consequences from reduced quality of life and perception of physical health to haemorrhoids. An understanding of the effectiveness and safety of treatments for constipation in pregnancy is important for the clinician managing pregnant women.
Objectives: To assess the effectiveness and safety of interventions (pharmacological and non-pharmacological) for treating constipation in pregnancy.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2015), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (30 April 2015) and reference lists of retrieved studies.
Selection criteria: We considered all published, unpublished and ongoing randomised controlled trials (RCTs), cluster-RCTs and quasi-RCTs, evaluating interventions (pharmacological and non-pharmacological) for constipation in pregnancy. Cross-over studies were not eligible for inclusion in this review. Trials published in abstract form only (without full text publication) were not eligible for inclusion.We compared one intervention (pharmacological or non-pharmacological) against another intervention, placebo or no treatment.
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
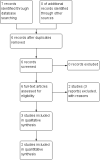
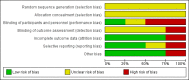
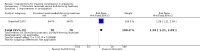
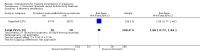
Main results: Four studies were included, but only two studies with a total of 180 women contributed data to this review. It was not clear whether they were RCTs or quasi-RCTs because the sequence generation was unclear. We classified the overall risk of bias of three studies as moderate and one study as high risk of bias. No meta-analyses were carried out due to insufficient data.There were no cluster-RCTs identified for inclusion. Comparisons were available for stimulant laxatives versus bulk-forming laxatives, and fibre supplementation versus no intervention. There were no data available for any other comparisons.During the review process we found that studies reported changes in symptoms in different ways. To capture all data available, we added a new primary outcome (improvement in constipation) - this new outcome was not prespecified in our published protocol. Stimulant laxatives versus bulk-forming laxativesNo data were identified for any of this review's prespecified primary outcomes: pain on defecation, frequency of stools and consistency of stools.Compared to bulk-forming laxatives, pregnant women who received stimulant laxatives had significantly more improvement in constipation (risk ratio (RR) 1.59, 95% confidence interval (CI) 1.21 to 2.09; 140 women, one study, moderate quality of evidence), but also significantly more abdominal discomfort (RR 2.33, 95% CI 1.15 to 4.73; 140 women, one study, low quality of evidence), and borderline difference in diarrhoea (RR 4.50, 95% CI 1.01 to 20.09; 140 women, one study, moderate quality of evidence). In addition, there was no significant difference in women's satisfaction (RR 1.06, 95% CI 0.77 to 1.46; 140 women, one study, moderate quality of evidence).No usable data were identified for any of this review's secondary outcomes: quality of life; dehydration; electrolyte imbalance; acute allergic reaction; or asthma. Fibre supplementation versus no interventionPregnant women who received fibre supplementation had significantly higher frequency of stools compared to no intervention (mean difference (MD) 2.24 times per week, 95% CI 0.96 to 3.52; 40 women, one study, moderate quality of evidence). Fibre supplementation was associated with improved stool consistency as defined by trialists (hard stool decreased by 11% to 14%, normal stool increased by 5% to 10%, and loose stool increased by 0% to 6%).No usable data were reported for either the primary outcomes of pain on defecation and improvement in constipation or any of this review's secondary outcomes as listed above. Quality Five outcomes were assessed with the GRADE software: improvement in constipation, frequency of stools, abdominal discomfort, diarrhoea and women's satisfaction. These were assessed to be of moderate quality except for abdominal discomfort which was assessed to be of low quality. The results should therefore be interpreted with caution. There were no data available for evaluation of pain on defecation or consistency of stools.
Authors' conclusions: There is insufficient evidence to comprehensively assess the effectiveness and safety of interventions (pharmacological and non-pharmacological) for treating constipation in pregnancy, due to limited data (few studies with small sample size and no meta-analyses). Compared with bulk-forming laxatives, stimulant laxatives appear to be more effective in improvement of constipation (moderate quality evidence), but are accompanied by an increase in diarrhoea (moderate quality evidence) and abdominal discomfort (low quality evidence) and no difference in women's satisfaction (moderate quality evidence). Additionally, fibre supplementation may increase frequency of stools compared with no intervention (moderate quality evidence), although these results were of moderate risk of bias.There were no data for a comparison of other types of interventions, such as osmotic laxatives, stool softeners, lubricant laxatives and enemas and suppositories.More RCTs evaluating interventions for treating constipation in pregnancy are needed. These should cover different settings and evaluate the effectiveness of various interventions (including fibre, osmotic, and stimulant laxatives) on improvement in constipation, pain on defecation, frequency of stools and consistency of stools.
Conflict of interest statement
Phassawan Rungsiprakarn: none known.
Malinee Laopaiboon: none known.
Ussanee S Sangkomkamhang: none known.
Pisake Lumbiganon: none known.
Jeremy J Pratt: none known.
Figures







Update of
- doi: 10.1002/14651858.CD011448
Similar articles
-
Pharmacological treatment for antipsychotic-related constipation.Cochrane Database Syst Rev. 2017 Jan 24;1(1):CD011128. doi: 10.1002/14651858.CD011128.pub2. Cochrane Database Syst Rev. 2017. PMID: 28116777 Free PMC article.
-
Interventions for preventing postpartum constipation.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011625. doi: 10.1002/14651858.CD011625.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Aug 5;8:CD011625. doi: 10.1002/14651858.CD011625.pub3. PMID: 26387487 Free PMC article. Updated.
-
Osmotic and stimulant laxatives for the management of childhood constipation.Cochrane Database Syst Rev. 2016 Aug 17;2016(8):CD009118. doi: 10.1002/14651858.CD009118.pub3. Cochrane Database Syst Rev. 2016. PMID: 27531591 Free PMC article.
-
Osmotic and stimulant laxatives for the management of childhood constipation.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD009118. doi: 10.1002/14651858.CD009118.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Aug 17;(8):CD009118. doi: 10.1002/14651858.CD009118.pub3. PMID: 22786523 Updated.
-
Interventions for heartburn in pregnancy.Cochrane Database Syst Rev. 2015 Sep 19;2015(9):CD011379. doi: 10.1002/14651858.CD011379.pub2. Cochrane Database Syst Rev. 2015. PMID: 26384956 Free PMC article.
Cited by
-
A Gender Perspective on Coloproctological Diseases: A Narrative Review on Female Disorders.J Clin Med. 2024 Oct 15;13(20):6136. doi: 10.3390/jcm13206136. J Clin Med. 2024. PMID: 39458086 Free PMC article. Review.
-
Pharmacological treatment for antipsychotic-related constipation.Cochrane Database Syst Rev. 2017 Jan 24;1(1):CD011128. doi: 10.1002/14651858.CD011128.pub2. Cochrane Database Syst Rev. 2017. PMID: 28116777 Free PMC article.
-
The effect of combined fig-Walnut syrup on functional constipation in pregnant women: a randomized controlled trial.Nutr Metab (Lond). 2025 Jan 17;22(1):3. doi: 10.1186/s12986-025-00895-3. Nutr Metab (Lond). 2025. PMID: 39825362 Free PMC article.
-
Haemorrhoidal disease in pregnancy: results from a self-assessment questionnaire administered by means of a social network.BMC Gastroenterol. 2024 May 2;24(1):150. doi: 10.1186/s12876-024-03228-5. BMC Gastroenterol. 2024. PMID: 38698334 Free PMC article.
-
Impact of Mediterranean Diet Adherence in Early Pregnancy on Nausea, Vomiting, and Constipation.Matern Child Health J. 2025 May;29(5):639-649. doi: 10.1007/s10995-025-04078-7. Epub 2025 Apr 7. Matern Child Health J. 2025. PMID: 40195164 Free PMC article.
References
References to studies included in this review
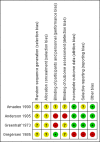
Amadeo 1990 {published data only}
-
- Amadeo A, Fatti F, Pellegrini V, Szijarto A. Controlled double blind study versus placebo of a formulation of glaucomannan and mineral salts in pregnant patients with constipation [Studio controllato in condizioni di doppia cecita' verso placebo di una preparazione a base di glucomannano e sali minerali in pazienti gravide con stipsi]. Rassegna Internazionale di Clinica e Terapia 1990;70:881‐90.
Anderson 1985 {published data only}
-
- Anderson AS, Whichelow MJ. Constipation during pregnancy: dietary fibre intake and the effect of fibre supplementation. Human Nutrition: Applied Nutrition 1985;39:202‐7. - PubMed
Greenhalf 1973 {published data only}
-
- Greenhalf JO, Leonard HSD. Laxatives in the treatment of constipation in pregnant and breast‐feeding mothers. Practitioner 1973;210:259‐63. - PubMed
Gregersen 1985 {published data only}
-
- Gregersen E. Constipation during pregnancy. Treatment with Dumovital fibre tablets. Ugeskrift for Laeger 1985;147:91‐3. - PubMed
References to studies excluded from this review
Browne 1957 {published data only}
Ghahramani 2013 {published data only}
-
- Ghahramani L. Evaluation the effect of Psyllium powder (oral herbal laxative) in prevention of constipation, hemorrhoids and anal fissure during pregnancy in pregnant women (3rd trimester) who will refer to Pregnancy Care Clinics in Shiraz, Iran during 1 year. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 4 January 2015] 2010.
-
- Ghahramani L, Hosseini S V, Rahimikazerooni S, Bananzadeh A M, Namavar Jahromi B, Samsam A, et al. The effect of oral Psyllium herbal laxative powder in prevention of hemorrhoids and anal fissure during pregnancy, a randomized double blind clinical trial. Annals of Colorectal Research 2013;1:23‐7.
Additional references
Acs 2002
-
- Acs N, Banhidy F, Puho EH, Czeizel AE. No association between severe constipation with related drug treatment in pregnant women and congenital abnormalities in their offspring: a population‐based case‐control study. Congenital Anomalies 2002;50(1):15‐20. - PubMed
Artal 2003
BNF 2010
-
- Royal Pharmaceutical Society. British National Formulary. 60th. London: BMJ Publishing Group Ltd and Royal Pharmaceutical Society, 2010.
Bradley 2007
-
- Bradley CS, Kennedy CM, Turcea AM, Rao SS, Nygaard IE. Constipation in pregnancy: prevalence, symptoms, and risk factors. Obstetrics and Gynecology 2007;110(6):1351‐7. - PubMed
Clemens 2013
-
- Clemens KE, Faust M, Jaspers B, Mikus G. Pharmacological treatment of constipation in palliative care. Current Opinion in Supportive and Palliative Care 2013;7(2):183‐91. - PubMed
Corazziari 2000
-
- Corazziari E, Badiali D, Bazzocchi G, Bassotti G, Roselli P, Mastropaolo G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF‐100) in the treatment of functional chronic constipation. Gut 2000;46(4):522‐6. - PMC - PubMed
Cullen 2007
-
- Cullen G, O'Donoghue D. Constipation and pregnancy. Best Practice & Research. Clinical Gastroenterology 2007;21(5):807‐18. - PubMed
Derbyshire 2006
Drossman 2006
-
- Drossman DA. Rome III: the new criteria. Chinese Journal of Digestive Diseases 2006;7(4):181‐5. - PubMed
GRADE 2014 [Computer program]
-
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Grossmann 2000
-
- Grossmann EM, Kaminski DL, Amon E, Longo WE. Idiopathic megarectum complicating pregnancy: report of a case. American Journal of Gastroenterology 2000;95(10):2969‐72. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irvine 2002
-
- Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health‐related quality of life in functional GI disorders: focus on constipation and resource utilization. American Journal of Gastroenterology 2002;97(8):1986‐93. - PubMed
Johnson 2014
-
- Johnson P, Mount K, Graziano S. Functional bowel disorders in pregnancy: effect on quality of life, evaluation and management. Acta Obstetricia et Gynecologica Scandinavica 2014;93(9):874‐9. - PubMed
Klaschik 2003
-
- Klaschik E, Nauck F, Ostgathe C. Constipation‐‐modern laxative therapy. Supportive Care in Cancer 2003;11(11):679‐85. - PubMed
Kyle 2006
-
- Kyle G. Assessment and treatment of older patients with constipation. Nursing Standard 2006;21(8):41‐6. - PubMed
Lawson 1985
-
- Lawson M, Kern F Jr, Everson GT. Gastrointestinal transit time in human pregnancy: prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology 1985;89(5):996‐9. - PubMed
Leung 2011
-
- Leung L, Riutta T, Kotecha J, Rosser W. Chronic constipation: an evidence‐based review. Journal of the American Board of Family Medicine 2011;24(4):436‐51. - PubMed
Longo 2010
Marlett 2002
-
- Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. Journal of the American Dietetic Association 2002;102(7):993‐1000. - PubMed
MIMS 2014
-
- MIMS. MIMS Drug Information System; senna. available from https://www.mims.com/resources/portal/common/document/mims/mimsau.htm (accessed 1 October 2014).
Moriarty 1992
Muller‐Lissner 1988
NICE 2008
-
- Antenatal Care. http://www.nice.org.uk/nicemedia/pdf/CG062PublicInfo.pdf (accessed 1 October 2014) 2008.
Pate 1995
-
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402‐7. - PubMed
Ponce 2008
-
- Ponce J, Martinez B, Fernandez A, Ponce M, Bastida G, Pla E, et al. Constipation during pregnancy: a longitudinal survey based on self‐reported symptoms and the Rome II criteria. European Journal of Gastroenterology & Hepatology 2008;20(1):56‐61. - PubMed
Portalatin 2012
Potter 2005
Prather 2004
-
- Prather CM. Pregnancy‐related constipation. Current Gastroenterology Reports 2004;6(5):402‐4. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. - PubMed
Shafe 2011
-
- Shafe AC, Lee S, Dalrymple JS, Whorwell PJ. The LUCK study: Laxative Usage in patients with GP‐diagnosed Constipation in the UK, within the general population and in pregnancy. An epidemiological study using the General Practice Research Database (GPRD). Therapeutic Advances in Gastroenterology 2011;4(6):343‐63. - PMC - PubMed
Trottier 2012
Twycross 2012
-
- Twycross R, Sykes N, Mihalyo M, Wilcock A. Stimulant laxatives and opioid‐induced constipation. Journal of Pain and Symptom Management 2012;43(2):306‐13. - PubMed
Tytgat 2003
-
- Tytgat GN, Heading RC, Muller‐Lissner S, Kamm MA, Scholmerich J, Berstad A, et al. Contemporary understanding and management of reflux and constipation in the general population and pregnancy: a consensus meeting. Alimentary Pharmacology and Therapeutics 2003;18(3):291‐301. - PubMed
Vaswani 1996
-
- Vaswani SK, Hamilton RG, Valentine MD, Adkinson NF Jr. Psyllium laxative‐induced anaphylaxis, asthma, and rhinitis. Allergy 1996;51(4):266‐8. - PubMed
Vazquez 2010
Wald 2003
-
- Wald A. Constipation, diarrhea, and symptomatic hemorrhoids during pregnancy. Gastroenterology Clinics of North America 2003;32(1):309‐22, vii. - PubMed
Wald 2007
-
- Wald A, Scarpignato C, Kamm MA, Mueller‐Lissner S, Helfrich I, Schuijt C, et al. The burden of constipation on quality of life: results of a multinational survey. Alimentary Pharmacology & Therapeutics 2007;26(2):227‐36. - PubMed
Xing 2001
-
- Xing JH, Soffer EE. Adverse effects of laxatives. Diseases of the Colon and Rectum 2001;44(8):1201‐9. - PubMed
References to other published versions of this review
Jewell 2001
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

