Role of extrahepatic UDP-glucuronosyltransferase 1A1: Advances in understanding breast milk-induced neonatal hyperbilirubinemia
- PMID: 26342858
- PMCID: PMC4708261
- DOI: 10.1016/j.taap.2015.08.018
Role of extrahepatic UDP-glucuronosyltransferase 1A1: Advances in understanding breast milk-induced neonatal hyperbilirubinemia
Abstract
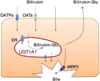
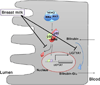
Newborns commonly develop physiological hyperbilirubinemia (also known as jaundice). With increased bilirubin levels being observed in breast-fed infants, breast-feeding has been recognized as a contributing factor for the development of neonatal hyperbilirubinemia. Bilirubin undergoes selective metabolism by UDP-glucuronosyltransferase (UGT) 1A1 and becomes a water soluble glucuronide. Although several factors such as gestational age, dehydration and weight loss, and increased enterohepatic circulation have been associated with breast milk-induced jaundice (BMJ), deficiency in UGT1A1 expression is a known cause of BMJ. It is currently believed that unconjugated bilirubin is metabolized mainly in the liver. However, recent findings support the concept that extrahepatic tissues, such as small intestine and skin, contribute to bilirubin glucuronidation during the neonatal period. We will review the recent advances made towards understanding biological and molecular events impacting BMJ, especially regarding the role of extrahepatic UGT1A1 expression.
Keywords: Bilirubin; Extra hepatic; Gilbert's Syndrome; Humanized UGT1 Mice; UDP-glucuronosyltransferase 1A1.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Prolonged unconjugated hyperbilirubinemia associated with breast milk and mutations of the bilirubin uridine diphosphate- glucuronosyltransferase gene.Pediatrics. 2000 Nov;106(5):E59. doi: 10.1542/peds.106.5.e59. Pediatrics. 2000. PMID: 11061796
-
Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice.J Pediatr. 2014 Jul;165(1):36-41.e1. doi: 10.1016/j.jpeds.2014.01.060. Epub 2014 Mar 17. J Pediatr. 2014. PMID: 24650397 Free PMC article.
-
Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus.Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):5024-9. doi: 10.1073/pnas.0913290107. Epub 2010 Mar 1. Proc Natl Acad Sci U S A. 2010. PMID: 20194756 Free PMC article.
-
Molecular genetic basis of Gilbert's syndrome.J Gastroenterol Hepatol. 1999 Oct;14(10):960-6. doi: 10.1046/j.1440-1746.1999.01984.x. J Gastroenterol Hepatol. 1999. PMID: 10530490 Review.
-
Function, genetic polymorphism, and transcriptional regulation of human UDP-glucuronosyltransferase (UGT) 1A1.Drug Metab Pharmacokinet. 2013;28(2):83-92. doi: 10.2133/dmpk.dmpk-12-rv-096. Epub 2012 Oct 23. Drug Metab Pharmacokinet. 2013. PMID: 23089802 Review.
Cited by
-
The Ontogeny of UDP-glucuronosyltransferase Enzymes, Recommendations for Future Profiling Studies and Application Through Physiologically Based Pharmacokinetic Modelling.Clin Pharmacokinet. 2019 Feb;58(2):189-211. doi: 10.1007/s40262-018-0681-2. Clin Pharmacokinet. 2019. PMID: 29862468 Review.
-
Structure and Protein-Protein Interactions of Human UDP-Glucuronosyltransferases.Front Pharmacol. 2016 Oct 24;7:388. doi: 10.3389/fphar.2016.00388. eCollection 2016. Front Pharmacol. 2016. PMID: 27822186 Free PMC article. Review.
-
Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption.Pharmaceutics. 2021 Jun 15;13(6):887. doi: 10.3390/pharmaceutics13060887. Pharmaceutics. 2021. PMID: 34203816 Free PMC article. Review.
-
Species differences in drug glucuronidation: Humanized UDP-glucuronosyltransferase 1 mice and their application for predicting drug glucuronidation and drug-induced toxicity in humans.Drug Metab Pharmacokinet. 2018 Feb;33(1):9-16. doi: 10.1016/j.dmpk.2017.10.002. Epub 2017 Oct 7. Drug Metab Pharmacokinet. 2018. PMID: 29079228 Free PMC article. Review.
-
Experimental models assessing bilirubin neurotoxicity.Pediatr Res. 2020 Jan;87(1):17-25. doi: 10.1038/s41390-019-0570-x. Epub 2019 Sep 7. Pediatr Res. 2020. PMID: 31493769 Review.
References
-
- Agrawal SK, Kumar P, Rathi R, Sharma N, Das R, Prasad R, Narang A. UGT1A1 gene polymorphisms in North Indian neonates presenting with unconjugated hyperbilirubinemia. Pediatr. Res. 2009;65:675–680. - PubMed
-
- Alonso EM, Whitington PF, Whitington SH, Rivard WA, Given G. Enterohepatic circulation of nonconjugated bilirubin in rats fed with human milk. J. Pediatr. 1991;118:425–430. - PubMed
-
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. - PubMed
-
- Aono S, Yamada Y, Keino H, Hanada N, Nakagawa T, Sasaoka Y, Yazawa T, Sato H, Koiwai O. Identification of defect in the genes for bilirubin UDP-glucuronosyl-transferase in a patient with Crigler-Najjar syndrome type II. Biochem. Biophys. Res. Commun. 1993;197:1239–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

