Vaccine Adjuvants: from 1920 to 2015 and Beyond
- PMID: 26343190
- PMCID: PMC4494348
- DOI: 10.3390/vaccines3020320
Vaccine Adjuvants: from 1920 to 2015 and Beyond
Abstract
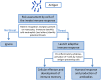
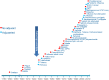
The concept of stimulating the body's immune response is the basis underlying vaccination. Vaccines act by initiating the innate immune response and activating antigen presenting cells (APCs), thereby inducing a protective adaptive immune response to a pathogen antigen. Adjuvants are substances added to vaccines to enhance the immunogenicity of highly purified antigens that have insufficient immunostimulatory capabilities, and have been used in human vaccines for more than 90 years. While early adjuvants (aluminum, oil-in-water emulsions) were used empirically, rapidly increasing knowledge on how the immune system interacts with pathogens means that there is increased understanding of the role of adjuvants and how the formulation of modern vaccines can be better tailored towards the desired clinical benefit. Continuing safety evaluation of licensed vaccines containing adjuvants/adjuvant systems suggests that their individual benefit-risk profile remains favorable. Adjuvants contribute to the initiation of the innate immune response induced by antigens; exemplified by inflammatory responses at the injection site, with mostly localized and short-lived effects. Activated effectors (such as APCs) then move to draining lymph nodes where they direct the type, magnitude and quality of the adaptive immune response. Thus, the right match of antigens and adjuvants can potentiate downstream adaptive immune responses, enabling the development of new efficacious vaccines. Many infectious diseases of worldwide significance are not currently preventable by vaccination. Adjuvants are the most advanced new technology in the search for new vaccines against challenging pathogens and for vulnerable populations that respond poorly to traditional vaccines.
Keywords: adaptive immune response; adjuvant; immune response; immunogenicity; innate immune response; safety; vaccine.
Figures




References
-
- Gross C.P., Sepkowitz K.A. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 1998;3:54–60. - PubMed
-
- Strugnell R., Zepp F., Cunningham A.L., Tantawhichien T. Vaccine antigens. Underst. Mod. Vaccines Perspect. Vaccinol. 2011;1:61–88. doi: 10.1016/j.pervac.2011.05.003. - DOI
-
- WHO Global Vaccine Action Plan 2011–2020. [(accessed on 23 October 2014)]. Available online: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011...
-
- Bonanni P., Santos J. Vaccine evolution. Underst. Mod. Vaccines Perspect. Vaccinol. 2011;1:1–24. doi: 10.1016/j.pervac.2011.05.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

