Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines
- PMID: 26343192
- PMCID: PMC4494344
- DOI: 10.3390/vaccines3020373
Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines
Abstract
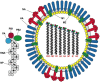
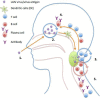
Influenza is a major respiratory pathogen causing annual outbreaks and occasional pandemics. Influenza vaccination is the major method of prophylaxis. Currently annual influenza vaccination is recommended for groups at high risk of complications from influenza infection such as pregnant women, young children, people with underlying disease and the elderly, along with occupational groups such a healthcare workers and farm workers. There are two main types of vaccines available: the parenteral inactivated influenza vaccine and the intranasal live attenuated influenza vaccine. The inactivated vaccines are licensed from 6 months of age and have been used for more than 50 years with a good safety profile. Inactivated vaccines are standardized according to the presence of the viral major surface glycoprotein hemagglutinin and protection is mediated by the induction of vaccine strain specific antibody responses. In contrast, the live attenuated vaccines are licensed in Europe for children from 2-17 years of age and provide a multifaceted immune response with local and systemic antibody and T cell responses but with no clear correlate of protection. Here we discuss the immunological immune responses elicited by the two vaccines and discuss future work to better define correlates of protection.
Keywords: Influenza; T-cells; antibodies; immunity; immunization; live attenuated vaccine; vaccination.
Figures

References
-
- Rumke H.C., Richardus J.H., Rombo L., Pauksens K., Plassmann G., Durand C., Devaster J.M., Dewe W., Oostvogels L. Selection of an adjuvant for seasonal influenza vaccine in elderly people: Modelling immunogenicity from a randomized trial. BMC Infect. Dis. 2013 doi: 10.1186/1471-2334-13-348. - DOI - PMC - PubMed
-
- Hoffmann E., Mahmood K., Chen Z., Yang C.F., Spaete J., Greenberg H.B., Herlocher M.L., Jin H., Kemble G. Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J. Virol. 2005;79:11014–11021. doi: 10.1128/JVI.79.17.11014-11021.2005. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

