Vitamin E supplementation in pregnancy
- PMID: 26343254
- PMCID: PMC8406700
- DOI: 10.1002/14651858.CD004069.pub3
Vitamin E supplementation in pregnancy
Abstract
Background: Vitamin E supplementation may help reduce the risk of pregnancy complications involving oxidative stress, such as pre-eclampsia. There is a need to evaluate the efficacy and safety of vitamin E supplementation in pregnancy.
Objectives: To assess the effects of vitamin E supplementation, alone or in combination with other separate supplements, on pregnancy outcomes, adverse events, side effects and use of health services.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2015) and reference lists of retrieved studies.
Selection criteria: All randomised or quasi-randomised controlled trials evaluating vitamin E supplementation in pregnant women. We excluded interventions using a multivitamin supplement that contained vitamin E.
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
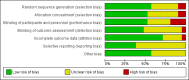
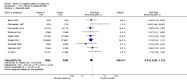
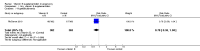
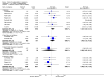
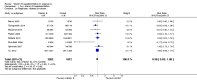
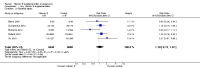
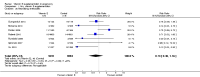
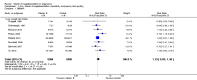
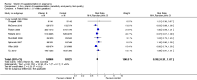
Main results: Twenty-one trials, involving 22,129 women were eligible for this review. Four trials did not contribute data. All of the remaining 17 trials assessed vitamin E in combination with vitamin C and/or other agents. Overall the risk of bias ranged from low to unclear to high; 10 trials were judged to be at low risk of bias, six trials to be at unclear risk of bias and five trials to be at high risk of bias. No clear difference was found between women supplemented with vitamin E in combination with other supplements during pregnancy compared with placebo for the risk of stillbirth (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.88 to 1.56, nine studies, 19,023 participants, I² = 0%; moderate quality evidence), neonatal death (RR 0.81, 95% CI 0.58 to 1.13, nine trials, 18,617 participants, I² = 0%), pre-eclampsia (average RR 0.91, 95% CI 0.79 to 1.06; 14 trials, 20,878 participants; I² = 48%; moderate quality evidence), preterm birth (average RR 0.98, 95% CI 0.88 to 1.09, 11 trials, 20,565 participants, I² = 52%; high quality evidence) or intrauterine growth restriction (RR 0.98, 95% CI 0.91 to 1.06, 11 trials, 20,202 participants, I² = 17%; high quality evidence). Women supplemented with vitamin E in combination with other supplements compared with placebo were at decreased risk of having a placental abruption (RR 0.64, 95% CI 0.44 to 0.93, seven trials, 14,922 participants, I² = 0%; high quality evidence). Conversely, supplementation with vitamin E was associated with an increased risk of self-reported abdominal pain (RR 1.66, 95% CI 1.16 to 2.37, one trial, 1877 participants) and term prelabour rupture of membranes (PROM) (average RR 1.77, 95% CI 1.37 to 2.28, two trials, 2504 participants, I² = 0%); however, there was no corresponding increased risk for preterm PROM (average RR 1.27, 95% CI 0.93 to 1.75, five trials, 1999 participants, I² = 66%; low quality evidence). There were no clear differences between the vitamin E and placebo or control groups for any other maternal or infant outcomes. There were no clear differing patterns in subgroups of women based on the timing of commencement of supplementation or baseline risk of adverse pregnancy outcomes. The GRADE quality of the evidence was high for preterm birth, intrauterine growth restriction and placental abruption, moderate for stillbirth and clinical pre-eclampsia, and low for preterm PROM.
Authors' conclusions: The data do not support routine vitamin E supplementation in combination with other supplements for the prevention of stillbirth, neonatal death, preterm birth, pre-eclampsia, preterm or term PROM or poor fetal growth. Further research is required to elucidate the possible role of vitamin E in the prevention of placental abruption. There was no convincing evidence that vitamin E supplementation in combination with other supplements results in other important benefits or harms.
Conflict of interest statement
Caroline Crowther and Alice Rumbold are Investigators on one of the included trials (Rumbold 2006). Decision about inclusion of this trial and extraction of data about this trial was undertaken by Erika Ota, Hiroyuki Hori and Celine Miyazaki.
Figures












































Update of
-
Vitamin E supplementation in pregnancy.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004069. doi: 10.1002/14651858.CD004069.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2015 Sep 07;(9):CD004069. doi: 10.1002/14651858.CD004069.pub3. PMID: 15846695 Updated.
References
References to studies included in this review
Anthony 1996 {published data only}
-
- Anthony J, Smith P, Mantel G, Johanson R. A randomised controlled trial of vitamin E supplementation in conservatively managed pre‐eclamptic patients. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8;Seattle, Washington, USA. 1996:193.
Beazley 2005 {published data only}
-
- Beazley D, Ahokas R, Livingston J, Griggs M, Scroggs M, Sibai B. Effects of vitamin c and e supplementation on total antioxidant status (tas) and 8‐isoprostane (ip) levels in women at high risk for preeclampsia [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S76. - PubMed
-
- Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2005; Vol. 192:520‐1. - PubMed
-
- Beazley D, Livingston J, Kao L, Sibai B. Vitamin c and e supplementation in women at high risk for preeclampsia: a double‐blind placebo controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S216. - PubMed
Borna 2005 {published data only}
-
- Borna S, Borna H, Daneshbodie B. Vitamins C and E in the latency period in women with preterm premature rupture of membranes. International Journal of Gynecology & Obstetrics 2005; Vol. 90:16‐20. - PubMed
Chappell 1999 {published data only}
-
- Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, et al. Effect of antioxidants on the occurrence of pre‐eclampsia in women at increased risk: a randomised trial. Lancet 1999;354(9181):810‐6. - PubMed
-
- Chappell LC, Seed PT, Kelly FJ, Briley A, Hunt BJ, Charnock‐Jones DS, et al. Vitamin C and E supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. American Journal of Obstetrics and Gynecology 2002;187(3):777‐84. - PubMed
Gulmezoglu 1997 {published data only}
-
- Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MM. Antioxidants in the treatment of severe pre‐eclampsia: an explanatory randomised controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(6):689‐96. - PubMed
-
- Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MM. Placental malondialdehyde and glutathione levels in a controlled trial of antioxidant treatment in severe preeclampsia. Hypertension in pregnancy 1996;15(3):287‐95.
Gungorduk 2014 {published data only}
-
- Gungorduk K, Asicioglu O, Gungorduk OC, Yildirim G, Besimoglu B, Ark C. Does vitamin C and vitamin E supplementation prolong the latency period before delivery following the preterm premature rupture of membranes? a randomized controlled study. American Journal of Perinatology 2014;31(3):195‐202. - PubMed
Huria 2010 {published data only}
-
- Huria A, Gupta P, Kumar D, Sharma MK. Vitamin C and vitamin E supplementation in pregnant women at risk for pre eclampsia: a randomized controlled trial. Internet Journal of Health 2010; Vol. 10, issue 2.
Kalpdev 2011 {published data only}
-
- Kalpdev A, Saha SC, Dhawan V. Vitamin C and E supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertension in Pregnancy 2011;30(4):447‐56. - PubMed
Mahdy 2004 {published data only}
-
- Mahdy ZA, Siraj HH, Azwar MH, Wahab MA, Khaza'ai H, Mutalib MSA, et al. The role of palm oil vitamin E in the prevention of pregnancy‐induced hypertension [abstract]. Hypertension in Pregnancy 2004; Vol. 23, issue Suppl 1:67.
McCance 2010 {published data only}
-
- Holmes V, Young I, Maresh M, Pearson D, Walker J, McCance D, et al. The diabetes and pre‐eclampsia intervention trial (DAPIT) [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):68. - PubMed
-
- Holmes VA, Young IS, Maresh MJA, Pearson DWM, Walker JD, McCance D and the DAPIT Study Group. The diabetes and pre‐eclampsia intervention trial. International Journal of Gynecology & Obstetrics 2004;87:66‐71. - PubMed
-
- Johnston PC, Powell LA, McCance DR, Pogue K, McMaster C, Gilchrist S, et al. Placental protein tyrosine nitration and MAPK in type 1 diabetic pre‐eclampsia: Impact of antioxidant vitamin supplementation. Journal of Diabetes & its Complications 2013;27(4):322‐7. - PubMed
Nasrolahi 2006 {published data only}
-
- Nasrolahi Sh, Alimohammady Sh, Zamani M. The effect of antioxidants (vitamin e and c) on preeclampsia in primiparous women. Journal of Gorgan University of Medical Sciences 2006; Vol. 8, issue 1:17‐21.
Poston 2006 {published data only}
-
- Duckworth S, Shennan A, Chappell L, Seed P, Briley A. Effect of antioxidant supplementation on pre‐labour rupture of the membranes. Archives of Disease in Childhood: Fetal and Neonatal Edition 2011;96:Fa75‐Fa76.
-
- Greenough A, Shaheen S, Shennan A, Seed P. Respiratory outcome in childhood of maternal vitamin C and E supplementation. American Journal of Respiratory and Critical Care Medicine 2009;179:A5975.
-
- Greenough A, Shaheen SO, Shennan A, Seed PT, Poston L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax 2010;65(11):998‐1003. - PubMed
-
- Manning L, Briley A, Poston L, Chappell L, Shennan A. Supplementation with vitamins C and E in pregnancy and liver function [abstract]. BJOG: an international journal of obstetrics and gynaecology 2007; Vol. 114, issue 8:1038.
-
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH, for the Vitamins in Pre‐eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre‐eclampsia (VIP trial): randomised placebo‐controlled trial. Lancet 2006; Vol. 367, issue 9517:1145‐54. - PubMed
Pressman 2003 {published data only}
-
- Pressman EK, Cavanaugh JL, Mingione M, Norkus EP, Woods JR. Effects of maternal antioxidant supplementation on maternal and fetal antioxidant levels: a randomized, double‐blind study. American Journal of Obstetrics and Gynecology 2003;189:1720‐5. - PubMed
Rivas 2000 {published data only}
-
- Rivas‐Echeverria CA, Echeverria Y, Molina L, Novoa D. Synergic use of aspirin, fish oil and vitamins C and E for the prevention of preeclampsia [abstract]. Hypertension in Pregnancy 2000;19(Suppl 1):30.
Roberts 2010 {published data only}
-
- Gandley R, Abramovici A. Prenatal vitamin C and e supplementation is associated with a reduction in placental abruption and preterm birth in smokers. Pregnancy Hypertension 2012;2(3):331‐2. - PubMed
-
- Hauth J, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. Vitamin C and E supplementation to prevent preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 2009; Vol. 201, issue 6 Suppl 1:S175‐S176.
Rumbold 2006 {published data only}
-
- Crowther CA, Dekker G, Haslam R, Robinson J. Australian collaborative trial of supplements with vitamin C and vitamin E for the prevention of pre‐eclampsia: a randomised trial. Personal Communication 2004.
-
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Australian collaborative trial of supplements (ACTS) with vitamin C and E for the prevention of pre‐eclampsia ‐ a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia 2006:180.
-
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, for the ACTS Study Group. Vitamins C and E and the risks of preeclampsia and perinatal complications. New England Journal of Medicine 2006; Vol. 354, issue 17:1796‐806. - PubMed
Sawhney 2000 {published data only}
-
- Sawhney H, Singhal S, Vasishta K, Majumbar S. Effect of vitamin E supplementation on lipid peroxide levels in preeclampsia [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA 2000;Book 5:31.
Shahraki 2006 {published data only}
-
- Shahraki AD. Effects of vitamin E, calcium carbonate and milk of magnesium on muscular cramps in pregnant women. Journal of Medical Sciences 2006; Vol. 6, issue 6:979‐83.
Spinnato 2007 {published data only}
-
- Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins‐Costa S, et al. The impact of prior preeclampsia on the risk of superimposed preeclampsia and other adverse pregnancy outcomes in patients with chronic hypertension. American Journal of Obstetrics and Gynecology 2011;204(4):345.e1‐345.e6. - PubMed
-
- Spinnato II J, Freire S, Pinto e Silva JLP, Rudge M, Martins‐Costa S, Koch M, et al. Antioxidant therapy and PROM. American Journal of Obstetrics and Gynecology 2007; Vol. 197, issue 6 Suppl 1:S196, Abstract no: 685.
-
- Spinnato II JA, Freire S, Pinto E Silva JL, Cunha Rudge MV, Martins‐Costa S, Koch MA, et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstetrics & Gynecology 2007;110(6):1311‐8. - PubMed
Villar 2009 {published data only}
-
- Villar J, Purwar M, Merialdi M, Zavaleta N, Ngoc N, Anthony J, et al. Effect of vitamin C & E supplementation of pregnant women at risk of preeclampsia plus low nutritional status: the WHO trial. Hypertension in Pregnancy 2008; Vol. 27, issue 4:501.
-
- Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre‐eclampsia in populations of low nutritional status from developing countries. BJOG: an international journal of obstetrics and gynaecology 2009; Vol. 116, issue 6:780‐8. - PubMed
-
- Villar J, Purwar M, Merialdi M, Zavaleta N, Tien NN, Anthony J. WHO randomized trial of vitamin C and E supplementation among women at high risk for preeclampsia and nutritional deficiency. American Journal of Obstetrics and Gynecology 2007; Vol. 197, issue 6 Suppl 1:S4, Abstract no: 8.
Xu 2010 {published data only}
-
- Fraser W, Xiong X, Poston L, Shennan A. Bi‐national randomized controlled trial of vitamin c and e supplementation of pregnancy [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):43.
-
- Xu H, Perez‐Cuevas R, Xiong X, Reyes H, Julien P, Smith G, et al. A international trials of vitamins C and E in the prevention of preeclampsia (INTAPP trial). American Journal of Obstetrics and Gynecology 2009; Vol. 201, issue 6 Suppl 1:S2. - PubMed
-
- Xu H, Perez‐Cuevas R, Xiong X, Reyes H, Roy C, Julien P, et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). American Journal of Obstetrics and Gynecology 2010; Vol. 202, issue 3:239.e1‐239.e10. - PubMed
References to studies excluded from this review
Bolisetty 2002 {published data only}
-
- Bolisetty S, Naidoo D, Lui K, Koh TH, Watson D, Whitehall J. Antenatal supplementation of antioxidant vitamins to reduce the oxidative stress at delivery ‐ a pilot study. Early Human Development 2002;67:47‐53. - PubMed
Clark 2012 {published data only}
-
- Clark J, Craig L, McNeill G, Smith N, Norrie J, Devereux G. A novel dietary intervention to optimize vitamin E intake of pregnant women to 15 mg/day. Journal of the Academy of Nutrition and Dietetics 2012;112(2):297‐301. - PubMed
Lietz 2001 {published data only}
-
- Lietz G, Henry CJK, Mulokozi G, Mugyabuso JKL, Ballart A, Ndossi GD, et al. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. American Journal of Clinical Nutrition 2001;74:501‐9. - PubMed
Moldenhauer 2002 {published data only}
-
- Moldenhauer J, Guo S, Liang R, Prada J. Dietary intake levels of the antioxidants vitamin c and vitamin e are adequately achieved with standard prenatal vitamin supplementation in high risk pregnancy groups [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S99.
Wibowo 2012 {published data only}
-
- Sezikawa A. Antioxidant supplementation in pregnant women with low antioxidant status. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 June 2007] 2007.
-
- Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Idriansyah L, Fitriana I. Antioxidant supplementation in pregnant women with low antioxidant status. Journal of Obstetrics and Gynaecology Research 2012;38(9):1152‐61. [PUBMED: 22563751] - PubMed
References to studies awaiting assessment
Tan 1997 {published data only}
-
- Tan YL, Han BF, Cheng YZ, Lin ML, Huang JX, Hou SY, et al. The prevention of vitamin E in severe pregnancy induced hypertension syndrome. Journal of Practice Obstetrics and Gynecology 1997;13(6):313‐4.
Additional references
Basaran 2010
-
- Basaran A, Basaran M, Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: a systematic review and meta‐analysis. Obstetrical & Gynecological Survey 2010;65(10):653‐67. - PubMed
Bendich 1993
-
- Bendich A, Machlin LJ. The safety of oral intake of vitamin E: data from clinical studies from 1986‐1991. In: Packer L, Fuchs J editor(s). Vitamin E in Health and Disease. New York: Marcel Dekker, 1993:411‐6.
Bjelakovic 2012
Bjelakovic 2013
-
- Bjelakovic G, Nikolova D, Gluud C. Meta‐regression analyses, meta‐analyses, and trial sequential analyses of the effects of supplementation with beta‐carotene, vitamin A, and vitamin E singly or in different combinations on all‐cause mortality: do we have evidence for lack of harm?. PLoS One 2013;8(9):e74558. - PMC - PubMed
Conde‐Agudelo 2011
-
- Conde‐Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2011;204(6):503.e1‐503.e12. - PMC - PubMed
Devaraj 1997
-
- Devaraj S, Adams‐Huet B, Fuller CJ, Jialal I. Dose‐response comparison of RRR‐alpha‐tocopherol and all‐racemic alpha‐tocopherol on LDL oxidation. Arteriosclerosis, Thrombosis and Vascular Biology 1997;17(10):2273‐9. - PubMed
GRADE 2014 [Computer program]
-
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Gross 1982
-
- Gross SJ, Landaw SA. The effect of vitamin E on red cell hemolysis and bilirubinaemia. Annals of the New York Academy of Science 1982;393:315‐22. - PubMed
Hanson 1993
-
- Hanson U, Persson B. Outcome of pregnancies complicated by type 1 insulin‐dependent diabetes in Sweden: acute pregnancy complications, neonatal mortality and morbidity. American Journal of Perinatology 1993;10(4):330‐3. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Incebiyik 2015
-
- Incebiyik A, Vural M, Camuzcuoglu A, Camuzcuoglu H, Hilali NG, Taskin A, et al. Comparison of tissue prolidase enzyme activity and serum oxidative stress level between pregnant women with placental abruption and those with a healthy pregnancy. Archives of Gynecology and Obstetrics 2015;291(4):805‐9. - PubMed
IOM 2000
-
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academy Press, 2000. - PubMed
Johnson 1985
-
- Johnson L, Bowen FW Jr, Abbasi S, Herrmann N, Weston M, Sacks L, et al. Relationship of prolonged pharmacologic serum levels of vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birthweight 1,500 grams or less. Pediatrics 1985;75:619‐38. - PubMed
Kingdom 2000
-
- Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. European Journal of Obstetrics & Gynecology and Reproductive Biology 2000;92:35‐43. - PubMed
NHMRC 2006
-
- NHMRC. Nutrient Reference Values for Australia and New Zealand. Canberra, Australia: Commonwealth of Australia, 2006.
Packer 1979
-
- Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979;278:737‐8. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1990
-
- Roberts DCK. Vitamin E. In: Truswell AS, Dreosti IE, English RM, Palmer N, Rutihauser IHE editor(s). Recommended Nutrient Intakes, Australian Papers. Sydney: Australian Professional Publications, 1990:158‐73.
Rumbold 2005a
Rumbold 2005b
Saugstad 1988
-
- Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatric Research 1988;23:143‐50. - PubMed
Saugstad 2001
-
- Saugstad OD. Update on oxygen radical disease in neonatology. Current Opinion in Obstetrics and Gynecology 2001;13(2):147‐53. - PubMed
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. - PubMed
Sharma 1985
-
- Sharma SC, Walzman M, Bonnar J, Molloy A. Blood ascorbic acid and histamine levels in patients with placental bleeding. Human Nutrition. Clinical Nutrition 1985;39(3):233‐8. - PubMed
Sharma 1986
-
- Sharma SC, Bonnar J, Dóstalóva L. Comparison of blood levels of vitamin A, beta‐carotene and vitamin E in abruptio placentae with normal pregnancy. International Journal for Vitamin and Nutrition Research 1986;56(1):3‐9. - PubMed
Woods 2001
-
- Woods JR Jr, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes?. American Journal of Obstetrics and Gynecology 2001;185(1):5‐10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

