Rules for biocatalyst and reaction engineering to implement effective, NAD(P)H-dependent, whole cell bioreductions
- PMID: 26343336
- PMCID: PMC5414839
- DOI: 10.1016/j.biotechadv.2015.08.006
Rules for biocatalyst and reaction engineering to implement effective, NAD(P)H-dependent, whole cell bioreductions
Abstract
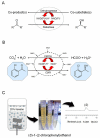
Access to chiral alcohols of high optical purity is today frequently provided by the enzymatic reduction of precursor ketones. However, bioreductions are complicated by the need for reducing equivalents in the form of NAD(P)H. The high price and molecular weight of NAD(P)H necessitate in situ recycling of catalytic quantities, which is mostly accomplished by enzymatic oxidation of a cheap co-substrate. The coupled oxidoreduction can be either performed by free enzymes in solution or by whole cells. Reductase selection, the decision between cell-free and whole cell reduction system, coenzyme recycling mode and reaction conditions represent design options that strongly affect bioreduction efficiency. In this paper, each option was critically scrutinized and decision rules formulated based on well-described literature examples. The development chain was visualized as a decision-tree that can be used to identify the most promising route towards the production of a specific chiral alcohol. General methods, applications and bottlenecks in the set-up are presented and key experiments required to "test" for decision-making attributes are defined. The reduction of o-chloroacetophenone to (S)-1-(2-chlorophenyl)ethanol was used as one example to demonstrate all the development steps. Detailed analysis of reported large scale bioreductions identified product isolation as a major bottleneck in process design.
Keywords: Chiral alcohol; Cost analysis; Decision tree for bioreduction development; Design of Escherichia coli whole cell catalysts; Limitations of whole cell reductions; Scale-up.
Copyright © 2015. Published by Elsevier Inc.
Figures
Similar articles
-
Scale-up and intensification of (S)-1-(2-chlorophenyl)ethanol bioproduction: economic evaluation of whole cell-catalyzed reduction of o-chloroacetophenone.Biotechnol Bioeng. 2013 Aug;110(8):2311-5. doi: 10.1002/bit.24896. Epub 2013 Mar 31. Biotechnol Bioeng. 2013. PMID: 23475609
-
Pushing the limits: Cyclodextrin-based intensification of bioreductions.J Biotechnol. 2021 Jan 10;325:57-64. doi: 10.1016/j.jbiotec.2020.11.017. Epub 2020 Nov 18. J Biotechnol. 2021. PMID: 33220340
-
Host cell and expression engineering for development of an E. coli ketoreductase catalyst: enhancement of formate dehydrogenase activity for regeneration of NADH.Microb Cell Fact. 2012 Jan 11;11:7. doi: 10.1186/1475-2859-11-7. Microb Cell Fact. 2012. PMID: 22236335 Free PMC article.
-
Biocatalytic ketone reduction: a green and efficient access to enantiopure alcohols.Biotechnol Adv. 2012 Nov-Dec;30(6):1279-88. doi: 10.1016/j.biotechadv.2011.10.007. Epub 2011 Oct 30. Biotechnol Adv. 2012. PMID: 22079798 Review.
-
Recent advances in enzymatic oxidation of alcohols.Curr Opin Chem Biol. 2018 Apr;43:77-86. doi: 10.1016/j.cbpa.2017.12.001. Epub 2017 Dec 16. Curr Opin Chem Biol. 2018. PMID: 29258054 Review.
Cited by
-
Production of semi-biosynthetic nepetalactone in yeast.J Ind Microbiol Biotechnol. 2019 Oct;46(9-10):1365-1370. doi: 10.1007/s10295-019-02199-x. Epub 2019 Jun 5. J Ind Microbiol Biotechnol. 2019. PMID: 31165969 Free PMC article.
-
Cyanobacteria-Mediated Light-Driven Biotransformation: The Current Status and Perspectives.ACS Omega. 2023 Oct 31;8(45):42062-42071. doi: 10.1021/acsomega.3c05407. eCollection 2023 Nov 14. ACS Omega. 2023. PMID: 38024730 Free PMC article. Review.
-
Reductive enzymatic dynamic kinetic resolution affording 115 g/L (S)-2-phenylpropanol.BMC Biotechnol. 2021 Oct 11;21(1):58. doi: 10.1186/s12896-021-00715-5. BMC Biotechnol. 2021. PMID: 34635076 Free PMC article.
-
Two novel cyanobacterial α-dioxygenases for the biosynthesis of fatty aldehydes.Appl Microbiol Biotechnol. 2022 Jan;106(1):197-210. doi: 10.1007/s00253-021-11724-x. Epub 2021 Dec 9. Appl Microbiol Biotechnol. 2022. PMID: 34882252 Free PMC article.
-
Characterization of a Carbonyl Reductase from Rhodococcus erythropolis WZ010 and Its Variant Y54F for Asymmetric Synthesis of (S)-N-Boc-3-Hydroxypiperidine.Molecules. 2018 Nov 28;23(12):3117. doi: 10.3390/molecules23123117. Molecules. 2018. PMID: 30487432 Free PMC article.
References
-
- Alphand V, Carrea G, Wohlgemuth R, Furstoss R, Woodley JM. Towards large-scale synthetic applications of Baeyer–Villiger monooxygenases. Trends Biotechnol. 2003;21:318–323. - PubMed
-
- Baldwin CVF, Wohlgemuth R, Woodley JM. The first 200-L scale asymmetric Baeyer–Villiger oxidation using a whole-cell biocatalyst. Org. Process. Res. Dev. 2008;12:660–665.
-
- Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004;22:1399–1408. - PubMed
-
- Blank LM, Ebert BE, Bühler B, Schmid A. Metabolic capacity estimation of Escherichia coli as a platform for redox biocatalysis: constraint-based modeling and experimental verification. Biotechnol. Bioeng. 2008;100:1050–1065. - PubMed
-
- Botes AL, Harvig D, van Dyk MS, Sarvary I, Frejd T, Katz M, Hahn-Hägerdal B, Gorwa-Grauslund MF. Screening of yeast species for the stereo-selective reduction of bicyclo[2.2.2]octane-2,6-dione. J. Chem. Soc. Perkin Trans. 2002;1:1111–1114.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

