Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP
- PMID: 26343373
- PMCID: PMC4652984
- DOI: 10.18632/oncotarget.4771
Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP
Abstract
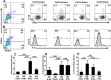
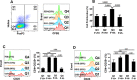
Regulatory T cells (Tregs) are key players of immune regulation/dysregulation both in physiological and pathophysiological settings. Despite significant advances in understanding Treg function, there is still a pressing need to define reliable and specific markers that can distinguish different Treg subpopulations. Herein we show for the first time that markers of activated Tregs [latency associated peptide (LAP) and glycoprotein A repetitions predominant (GARP, or LRRC32)] are expressed on CD4+FoxP3- T cells expressing Helios (FoxP3-Helios+) in the steady state. Following TCR activation, GARP/LAP are up-regulated on CD4+Helios+ T cells regardless of FoxP3 expression (FoxP3+/-Helios+). We show that CD4+GARP+/-LAP+ Tregs make IL-10 immunosuppressive cytokine but not IFN-γ effector cytokine. Further characterization of FoxP3/Helios subpopulations showed that FoxP3+Helios+ Tregs proliferate in vitro significantly less than FoxP3+Helios- Tregs upon TCR stimulation. Unlike FoxP3+Helios- Tregs, FoxP3+Helios+ Tregs secrete IL-10 but not IFN-γ or IL-2, confirming they are bona fide Tregs with immunosuppressive characteristics. Taken together, Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP, and FoxP3+Helios+ Tregs have more suppressive characteristics, compared with FoxP3+Helios- Tregs. Our work implies that therapeutic modalities for treating autoimmune and inflammatory diseases, allergies and graft rejection should be designed to induce and/or expand FoxP3+Helios+ Tregs, while therapies against cancers or infectious diseases should avoid such expansion/induction.
Keywords: FoxP3; GARP/LAP; Helios; Immune response; Immunity; Immunology and Microbiology Section; regulatory T cells.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



Similar articles
-
Combining FoxP3 and Helios with GARP/LAP markers can identify expanded Treg subsets in cancer patients.Oncotarget. 2016 Mar 22;7(12):14083-94. doi: 10.18632/oncotarget.7334. Oncotarget. 2016. PMID: 26885615 Free PMC article.
-
Heterogeneity in FoxP3- and GARP/LAP-Expressing T Regulatory Cells in an HLA Class II Transgenic Murine Model of Necrotizing Soft Tissue Infections by Group A Streptococcus.Infect Immun. 2018 Nov 20;86(12):e00432-18. doi: 10.1128/IAI.00432-18. Print 2018 Dec. Infect Immun. 2018. PMID: 30224551 Free PMC article.
-
IL-1R1 is expressed on both Helios(+) and Helios(-) FoxP3(+) CD4(+) T cells in the rheumatic joint.Clin Exp Immunol. 2015 Oct;182(1):90-100. doi: 10.1111/cei.12668. Epub 2015 Jul 30. Clin Exp Immunol. 2015. PMID: 26076982 Free PMC article.
-
Coexpression of Helios in Foxp3+ Regulatory T Cells and Its Role in Human Disease.Dis Markers. 2021 Jun 22;2021:5574472. doi: 10.1155/2021/5574472. eCollection 2021. Dis Markers. 2021. PMID: 34257746 Free PMC article. Review.
-
Helios: still behind the clouds.Immunology. 2019 Nov;158(3):161-170. doi: 10.1111/imm.13115. Epub 2019 Oct 13. Immunology. 2019. PMID: 31517385 Free PMC article. Review.
Cited by
-
Regulatory T Cells in Pregnancy: It Is Not All About FoxP3.Front Immunol. 2020 Jun 23;11:1182. doi: 10.3389/fimmu.2020.01182. eCollection 2020. Front Immunol. 2020. PMID: 32655556 Free PMC article. Review.
-
Effect of pembrolizumab on CD4+ CD25+ , CD4+ LAP+ and CD4+ TIM-3+ T cell subsets.Clin Exp Immunol. 2019 Jun;196(3):345-352. doi: 10.1111/cei.13264. Epub 2019 Feb 17. Clin Exp Immunol. 2019. PMID: 30693485 Free PMC article.
-
Role of α-fetoprotein in differentiation of regulatory T lymphocytes.Dokl Biol Sci. 2017 Nov;477(1):248-251. doi: 10.1134/S0012496617060084. Epub 2018 Jan 4. Dokl Biol Sci. 2017. PMID: 29299806
-
T-cell expression of AhR inhibits the maintenance of pTreg cells in the gastrointestinal tract in acute GVHD.Blood. 2017 Jul 20;130(3):348-359. doi: 10.1182/blood-2016-08-734244. Epub 2017 May 26. Blood. 2017. PMID: 28550042 Free PMC article.
-
Blockade of PD-1, PD-L1, and TIM-3 Altered Distinct Immune- and Cancer-Related Signaling Pathways in the Transcriptome of Human Breast Cancer Explants.Genes (Basel). 2020 Jun 25;11(6):703. doi: 10.3390/genes11060703. Genes (Basel). 2020. PMID: 32616706 Free PMC article.
References
-
- Elkord E, Alcantar-Orozco EM, Dovedi SJ, Tran DQ, Hawkins RE, Gilham DE. T regulatory cells in cancer: recent advances and therapeutic potential. Expert Opin Biol Ther. 2010;10:1573–1586. - PubMed
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. - PubMed
-
- von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12:51–63. - PubMed
-
- Chaudhary B, Abd Al Samid M, al-Ramadi BK, Elkord E. Phenotypic alterations, clinical impact and therapeutic potential of regulatory T cells in cancer. Expert Opin Biol Ther. 2014;14:931–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

