Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses
- PMID: 26343579
- PMCID: PMC4578813
- DOI: 10.1016/j.cell.2015.08.027
Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses
Erratum in
- Cell. 2015 Oct 8;163(2):523
Abstract
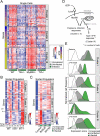
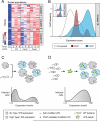
Encounters between immune cells and invading bacteria ultimately determine the course of infection. These interactions are usually measured in populations of cells, masking cell-to-cell variation that may be important for infection outcome. To characterize the gene expression variation that underlies distinct infection outcomes and monitor infection phenotypes, we developed an experimental system that combines single-cell RNA-seq with fluorescent markers. Probing the responses of individual macrophages to invading Salmonella, we find that variation between individual infected host cells is determined by the heterogeneous activity of bacterial factors in individual infecting bacteria. We illustrate how variable PhoPQ activity in the population of invading bacteria drives variable host type I IFN responses by modifying LPS in a subset of bacteria. This work demonstrates a causative link between host and bacterial variability, with cell-to-cell variation between different bacteria being sufficient to drive radically different host immune responses. This co-variation has implications for host-pathogen dynamics in vivo.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Single-Cell Analysis: The Differences That Kill.Cell. 2015 Sep 10;162(6):1208-10. doi: 10.1016/j.cell.2015.08.053. Cell. 2015. PMID: 26359980
References
-
- Ackermann M, Stecher B, Freed NE, Songhet P, Hardt WD, Doebeli M. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. - PubMed
-
- Claudi B, Sprote P, Chirkova A, Personnic N, Zankl J, Schurmann N, Schmidt A, Bumann D. Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell. 2014;158:722–733. - PubMed
-
- Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol. 2006;61:795–809. - PubMed
-
- Diard M, Garcia V, Maier L, Remus-Emsermann MN, Regoes RR, Ackermann M, Hardt WD. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature. 2013;494:353–356. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

