Enhanced Vibrational Spectroscopies as Tools for Small Molecule Biosensing
- PMID: 26343666
- PMCID: PMC4610423
- DOI: 10.3390/s150921239
Enhanced Vibrational Spectroscopies as Tools for Small Molecule Biosensing
Abstract
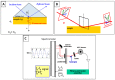
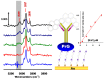
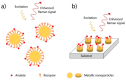
In this short summary we summarize some of the latest developments in vibrational spectroscopic tools applied for the sensing of (small) molecules and biomolecules in a label-free mode of operation. We first introduce various concepts for the enhancement of InfraRed spectroscopic techniques, including the principles of Attenuated Total Reflection InfraRed (ATR-IR), (phase-modulated) InfraRed Reflection Absorption Spectroscopy (IRRAS/PM-IRRAS), and Surface Enhanced Infrared Reflection Absorption Spectroscopy (SEIRAS). Particular attention is put on the use of novel nanostructured substrates that allow for the excitation of propagating and localized surface plasmon modes aimed at operating additional enhancement mechanisms. This is then be complemented by the description of the latest development in Surface- and Tip-Enhanced Raman Spectroscopies, again with an emphasis on the detection of small molecules or bioanalytes.
Keywords: IRRAS; SERS; biosensors; enhanced spectroscopies; infrared; plasmonic.
Figures






References
-
- Homola J., Yee S.S., Gauglitz G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999;54:3–15. doi: 10.1016/S0925-4005(98)00321-9. - DOI
-
- Nagl S., Schaeferling M., Wolfbeis O.S. Fluorescence analysis in microarray technology. Microchim. Acta. 2005;151:1–21. doi: 10.1007/s00604-005-0393-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

