Development of Biodegradable Polycation-Based Inhalable Dry Gene Powders by Spray Freeze Drying
- PMID: 26343708
- PMCID: PMC4588198
- DOI: 10.3390/pharmaceutics7030233
Development of Biodegradable Polycation-Based Inhalable Dry Gene Powders by Spray Freeze Drying
Abstract
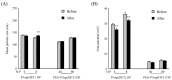
In this study, two types of biodegradable polycation (PAsp(DET) homopolymer and PEG-PAsp(DET) copolymer) were applied as vectors for inhalable dry gene powders prepared by spray freeze drying (SFD). The prepared dry gene powders had spherical and porous structures with a 5~10-μm diameter, and the integrity of plasmid DNA could be maintained during powder production. Furthermore, it was clarified that PEG-PAsp(DET)-based dry gene powder could more sufficiently maintain both the physicochemical properties and in vitro gene transfection efficiencies of polyplexes reconstituted after powder production than PAsp(DET)-based dry gene powder. From an in vitro inhalation study using an Andersen cascade impactor, it was demonstrated that the addition of l-leucine could markedly improve the inhalation performance of dry powders prepared by SFD. Following pulmonary delivery to mice, both PAsp(DET)- and PEG-PAsp(DET)-based dry gene powders could achieve higher gene transfection efficiencies in the lungs compared with a chitosan-based dry gene powder previously reported by us.
Keywords: biodegradable polycations; dry powder inhalers (DPIs); porous particles; pulmonary gene transfection; spray freeze drying (SFD).
Figures








Similar articles
-
Development of New Formulation Dry Powder for Pulmonary Delivery Using Amino Acids to Improve Stability.Biol Pharm Bull. 2016;39(3):394-400. doi: 10.1248/bpb.b15-00822. Epub 2015 Dec 25. Biol Pharm Bull. 2016. PMID: 26725531
-
Optimized pulmonary gene transfection in mice by spray-freeze dried powder inhalation.J Control Release. 2010 Jun 1;144(2):221-6. doi: 10.1016/j.jconrel.2010.02.018. Epub 2010 Feb 22. J Control Release. 2010. PMID: 20184930
-
Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity.J Control Release. 2018 Jun 10;279:99-113. doi: 10.1016/j.jconrel.2018.04.003. Epub 2018 Apr 6. J Control Release. 2018. PMID: 29627404
-
Spray Freeze Drying of Biologics: A Review and Applications for Inhalation Delivery.Pharm Res. 2023 May;40(5):1115-1140. doi: 10.1007/s11095-022-03442-4. Epub 2022 Dec 1. Pharm Res. 2023. PMID: 36456666 Review.
-
Spray drying: Inhalable powders for pulmonary gene therapy.Biomater Adv. 2022 Feb;133:112601. doi: 10.1016/j.msec.2021.112601. Epub 2021 Dec 9. Biomater Adv. 2022. PMID: 35527158 Review.
Cited by
-
Engineering Inhalable Therapeutic Particles: Conventional and Emerging Approaches.Pharmaceutics. 2023 Nov 30;15(12):2706. doi: 10.3390/pharmaceutics15122706. Pharmaceutics. 2023. PMID: 38140047 Free PMC article. Review.
-
Stabilization of Deformable Nanovesicles Based on Insulin-Phospholipid Complex by Freeze-Drying.Pharmaceutics. 2019 Oct 16;11(10):539. doi: 10.3390/pharmaceutics11100539. Pharmaceutics. 2019. PMID: 31623287 Free PMC article.
-
Physical stability of dry powder inhaler formulations.Expert Opin Drug Deliv. 2020 Jan;17(1):77-96. doi: 10.1080/17425247.2020.1702643. Epub 2019 Dec 13. Expert Opin Drug Deliv. 2020. PMID: 31815554 Free PMC article. Review.
-
Dry Powder for Pulmonary Delivery: A Comprehensive Review.Pharmaceutics. 2020 Dec 28;13(1):31. doi: 10.3390/pharmaceutics13010031. Pharmaceutics. 2020. PMID: 33379136 Free PMC article. Review.
-
Stability of Naked Nucleic Acids under Physical Treatment and Powder Formation: Suitability for Development as Dry Powder Formulations for Inhalation.Pharmaceutics. 2023 Dec 16;15(12):2786. doi: 10.3390/pharmaceutics15122786. Pharmaceutics. 2023. PMID: 38140126 Free PMC article.
References
-
- Geiger J, Aneja M.K., Rudolph C. Vectors for pulmonary gene therapy. Int. J. Pharm. 2010;390:84–88. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

