MicroRNAs and Osteolytic Bone Metastasis: The Roles of MicroRNAs in Tumor-Induced Osteoclast Differentiation
- PMID: 26343739
- PMCID: PMC4600156
- DOI: 10.3390/jcm4091741
MicroRNAs and Osteolytic Bone Metastasis: The Roles of MicroRNAs in Tumor-Induced Osteoclast Differentiation
Abstract
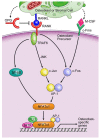
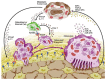
Osteolytic bone metastasis frequently occurs in the later stages of breast, lung, and several other cancers. Osteoclasts, the only cells that resorb bone, are hijacked by tumor cells, which break down bone remodeling systems. As a result, osteolysis occurs and may cause patients to suffer bone fractures, pain, and hypercalcemia. It is important to understand the mechanism of bone metastasis to establish new cancer therapies. MicroRNAs are small, noncoding RNAs that are involved in various biological processes, including cellular differentiation, proliferation, apoptosis, and tumorigenesis. MicroRNAs have significant clinical potential, including their use as new therapeutic targets and disease-specific biomarkers. Recent studies have revealed that microRNAs are involved in osteoclast differentiation and osteolytic bone metastasis. In this review focusing on microRNAs, the author discusses the roles of microRNAs in osteoclastogenesis and osteolytic bone metastasis.
Keywords: Bone Metastasis; Exosomes; Extracellular Vesicles; MicroRNAs; Osteoclasts.
Figures
Similar articles
-
Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis.Cancer Cell. 2013 Oct 14;24(4):542-56. doi: 10.1016/j.ccr.2013.09.008. Cancer Cell. 2013. PMID: 24135284 Free PMC article.
-
Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis.Gene. 2018 Aug 5;666:116-122. doi: 10.1016/j.gene.2018.05.008. Epub 2018 May 3. Gene. 2018. PMID: 29730429
-
The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line.Cancer Res. 2005 Jun 1;65(11):4929-38. doi: 10.1158/0008-5472.CAN-04-4458. Cancer Res. 2005. PMID: 15930315
-
MicroRNAs: Potential Biomarkers and Therapeutic Targets for Alveolar Bone Loss in Periodontal Disease.Int J Mol Sci. 2016 Aug 11;17(8):1317. doi: 10.3390/ijms17081317. Int J Mol Sci. 2016. PMID: 27529224 Free PMC article. Review.
-
P62: An emerging oncotarget for osteolytic metastasis.J Bone Oncol. 2016 Feb 3;5(1):30-7. doi: 10.1016/j.jbo.2016.01.003. eCollection 2016 Mar. J Bone Oncol. 2016. PMID: 26998424 Free PMC article. Review.
Cited by
-
Lung Cancer Cells Derived Circulating miR-21 Promotes Differentiation of Monocytes into Osteoclasts.Onco Targets Ther. 2020 Mar 31;13:2643-2656. doi: 10.2147/OTT.S232876. eCollection 2020. Onco Targets Ther. 2020. PMID: 32280240 Free PMC article.
-
miR‑203 contributes to pre‑eclampsia via inhibition of VEGFA expression.Mol Med Rep. 2018 Apr;17(4):5627-5634. doi: 10.3892/mmr.2018.8558. Epub 2018 Feb 2. Mol Med Rep. 2018. PMID: 29436641 Free PMC article.
-
Icariin ameliorates dexamethasone‑induced bone deterioration in an experimental mouse model via activation of microRNA‑186 inhibition of cathepsin K.Mol Med Rep. 2018 Jan;17(1):1633-1641. doi: 10.3892/mmr.2017.8065. Epub 2017 Nov 15. Mol Med Rep. 2018. PMID: 29257214 Free PMC article.
-
Targeting RHAMM in Cancer: Crosstalk with Non-Coding RNAs and Emerging Therapeutic Strategies Including Peptides, Oligomers, Antibodies, and Vaccines.Int J Mol Sci. 2025 Jul 25;26(15):7198. doi: 10.3390/ijms26157198. Int J Mol Sci. 2025. PMID: 40806330 Free PMC article. Review.
-
Osteoclast Signal Transduction During Bone Metastasis Formation.Front Cell Dev Biol. 2020 Jun 19;8:507. doi: 10.3389/fcell.2020.00507. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32637413 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

