Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling
- PMID: 26344101
- PMCID: PMC4598280
- DOI: 10.1016/j.cmet.2015.07.028
Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling
Abstract
Pyruvate transport across the inner mitochondrial membrane is believed to be a prerequisite for gluconeogenesis in hepatocytes, which is important for the maintenance of normoglycemia during prolonged food deprivation but also contributes to hyperglycemia in diabetes. To determine the requirement for mitochondrial pyruvate import in gluconeogenesis, mice with liver-specific deletion of mitochondrial pyruvate carrier 2 (LS-Mpc2(-/-)) were generated. Loss of MPC2 impaired, but did not completely abolish, hepatocyte conversion of labeled pyruvate to TCA cycle intermediates and glucose. Unbiased metabolomic analyses of livers from fasted LS-Mpc2(-/-) mice suggested that alterations in amino acid metabolism, including pyruvate-alanine cycling, might compensate for the loss of MPC2. Indeed, inhibition of pyruvate-alanine transamination further reduced mitochondrial pyruvate metabolism and glucose production by LS-Mpc2(-/-) hepatocytes. These data demonstrate an important role for MPC2 in controlling hepatic gluconeogenesis and illuminate a compensatory mechanism for circumventing a block in mitochondrial pyruvate import.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

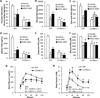

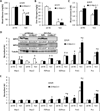


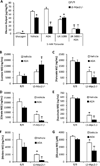
Comment in
-
Diabetes: Important role for MPC complex in hepatic gluconeogenesis.Nat Rev Endocrinol. 2015 Nov;11(11):629. doi: 10.1038/nrendo.2015.162. Epub 2015 Sep 22. Nat Rev Endocrinol. 2015. PMID: 26391977 No abstract available.
References
-
- Adams MD, Raman P, Judd RL. Comparative effects of englitazone and glyburide on gluconeogenesis and glycolysis in the isolated perfused rat liver. Biochemical pharmacology. 1998;55:1915–1920. - PubMed
-
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. The Journal of biological chemistry. 2006;281:19000–19008. - PMC - PubMed
-
- Casetta B, Tagliacozzi D, Shushan B, Federici G. Development of a method for rapid quantitation of amino acids by liquid chromatography-tandem mass spectrometry (LC-MSMS) in plasma. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2000;38:391–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R42 AA021228/AA/NIAAA NIH HHS/United States
- R01 DK078187/DK/NIDDK NIH HHS/United States
- P30 DK56341/DK/NIDDK NIH HHS/United States
- T32 DK007120/DK/NIDDK NIH HHS/United States
- T32 DK007296/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- R56 DK078184/DK/NIDDK NIH HHS/United States
- R01 DK104735/DK/NIDDK NIH HHS/United States
- R01 DK078184/DK/NIDDK NIH HHS/United States
- DK007120/DK/NIDDK NIH HHS/United States
- P30 DK20579/DK/NIDDK NIH HHS/United States
- T32 DK07296/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P01 DK058398/DK/NIDDK NIH HHS/United States
- P30 DK52574/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

