Picomolar sensitivity to inositol trisphosphate in Xenopus oocytes
- PMID: 26344104
- PMCID: PMC4708890
- DOI: 10.1016/j.ceca.2015.08.003
Picomolar sensitivity to inositol trisphosphate in Xenopus oocytes
Abstract
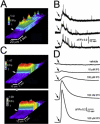
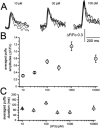
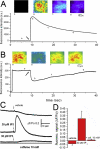
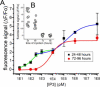
Ca(2+) liberation from the endoplasmic reticulum mediated by inositol trisphosphate receptor/channels (IP3Rs) in response to production of the second messenger IP3 regulates numerous signaling pathways. However, estimates of resting and physiologically relevant cytosolic concentrations of IP3 vary appreciably. Here we directly address this question, taking advantage of the large size of Xenopus oocytes to image Ca(2+) liberation evoked by bolus intracellular injections of known concentrations of IP3. Our principal finding is that IP3 evokes both global and local Ca(2+) signals in freshly isolated oocytes at concentrations as low as a few pM. A corollary is that basal, resting [IP3] must be even lower, given the absence of detectable Ca(2+) signals before injection. The dose/response curve for IP3-activation of Ca(2+) liberation suggests that freshly isolated oocytes express two distinct functional populations of IP3 receptors with EC50 values around 200 pM and tens of nM, whereas the high-affinity receptors are not apparent in oocytes examined later than about 3 days after isolation from the ovary.
Keywords: Calcium puffs; Calcium signaling; Inositol trisphosphate; Xenopus oocyte.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. - PubMed
-
- Parker I, Miledi R. Injection of inositol 1,3,4,5-tetrakisphosphate into Xenopus oocytes generates a chloride current dependent upon intracellular calcium. Proc R Soc Lond B Biol Sci. 1987;232:59–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

