Vector Design for Improved DNA Vaccine Efficacy, Safety and Production
- PMID: 26344110
- PMCID: PMC4494225
- DOI: 10.3390/vaccines1030225
Vector Design for Improved DNA Vaccine Efficacy, Safety and Production
Abstract
DNA vaccination is a disruptive technology that offers the promise of a new rapidly deployed vaccination platform to treat human and animal disease with gene-based materials. Innovations such as electroporation, needle free jet delivery and lipid-based carriers increase transgene expression and immunogenicity through more effective gene delivery. This review summarizes complementary vector design innovations that, when combined with leading delivery platforms, further enhance DNA vaccine performance. These next generation vectors also address potential safety issues such as antibiotic selection, and increase plasmid manufacturing quality and yield in exemplary fermentation production processes. Application of optimized constructs in combination with improved delivery platforms tangibly improves the prospect of successful application of DNA vaccination as prophylactic vaccines for diverse human infectious disease targets or as therapeutic vaccines for cancer and allergy.
Keywords: DNA vaccination; adjuvant; antibiotic-free; fermentation; immunization; innate immunity; non-viral; plasmid.
Figures

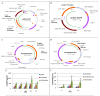

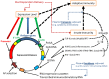
References
-
- Wang S., Parker C., Taaffe J., Solorzano A., Garcia-Sastre A., Lu S. Heterologous HA DNA vaccine prime-inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. doi: 10.1016/j.vaccine.2008.04.073. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

