Optimization of HIV-1 Envelope DNA Vaccine Candidates within Three Different Animal Models, Guinea Pigs, Rabbits and Cynomolgus Macaques
- PMID: 26344115
- PMCID: PMC4494233
- DOI: 10.3390/vaccines1030305
Optimization of HIV-1 Envelope DNA Vaccine Candidates within Three Different Animal Models, Guinea Pigs, Rabbits and Cynomolgus Macaques
Abstract
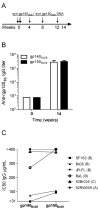
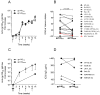
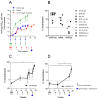
HIV-1 DNA vaccines have many advantageous features. Evaluation of HIV-1 vaccine candidates often starts in small animal models before macaque and human trials. Here, we selected and optimized DNA vaccine candidates through systematic testing in rabbits for the induction of broadly neutralizing antibodies (bNAb). We compared three different animal models: guinea pigs, rabbits and cynomolgus macaques. Envelope genes from the prototype isolate HIV-1 Bx08 and two elite neutralizers were included. Codon-optimized genes, encoded secreted gp140 or membrane bound gp150, were modified for expression of stabilized soluble trimer gene products, and delivered individually or mixed. Specific IgG after repeated i.d. inoculations with electroporation confirmed in vivo expression and immunogenicity. Evaluations of rabbits and guinea pigs displayed similar results. The superior DNA construct in rabbits was a trivalent mix of non-modified codon-optimized gp140 envelope genes. Despite NAb responses with some potency and breadth in guinea pigs and rabbits, the DNA vaccinated macaques displayed less bNAb activity. It was concluded that a trivalent mix of non-modified gp140 genes from rationally selected clinical isolates was, in this study, the best option to induce high and broad NAb in the rabbit model, but this optimization does not directly translate into similar responses in cynomolgus macaques.
Keywords: DNA vaccine; HIV-1; animal models; envelope; neutralizing antibodies.
Figures


References
-
- Binley J.M., Sanders R.W., Clas B., Schuelke N., Master A., Guo Y., Kajumo F., Anselma D.J., Maddon P.J., Olson W.C., et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 2000;74:627–643. doi: 10.1128/JVI.74.2.627-643.2000. - DOI - PMC - PubMed
-
- Sanders R.W., Vesanen M., Schuelke N., Master A., Schiffner L., Kalyanaraman R., Paluch M., Berkhout B., Maddon P.J., Olson W.C., et al. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 2002;76:8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

