Glycine receptor mechanism elucidated by electron cryo-microscopy
- PMID: 26344198
- PMCID: PMC4659708
- DOI: 10.1038/nature14853
Glycine receptor mechanism elucidated by electron cryo-microscopy
Abstract
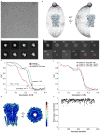
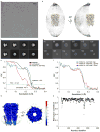
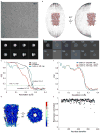
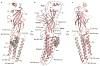
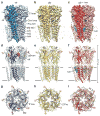
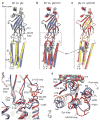
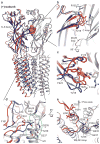
The strychnine-sensitive glycine receptor (GlyR) mediates inhibitory synaptic transmission in the spinal cord and brainstem and is linked to neurological disorders, including autism and hyperekplexia. Understanding of molecular mechanisms and pharmacology of glycine receptors has been hindered by a lack of high-resolution structures. Here we report electron cryo-microscopy structures of the zebrafish α1 GlyR with strychnine, glycine, or glycine and ivermectin (glycine/ivermectin). Strychnine arrests the receptor in an antagonist-bound closed ion channel state, glycine stabilizes the receptor in an agonist-bound open channel state, and the glycine/ivermectin complex adopts a potentially desensitized or partially open state. Relative to the glycine-bound state, strychnine expands the agonist-binding pocket via outward movement of the C loop, promotes rearrangement of the extracellular and transmembrane domain 'wrist' interface, and leads to rotation of the transmembrane domain towards the pore axis, occluding the ion conduction pathway. These structures illuminate the GlyR mechanism and define a rubric to interpret structures of Cys-loop receptors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



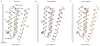
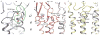

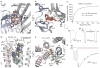

References
-
- Aprison MH, Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965;4:2075–83. - PubMed
-
- Curtis DR, Hosli L, Johnston GA. Inhibition of spinal neurons by glycine. Nature. 1967;215:1502–3. - PubMed
-
- Werman R, Davidoff RA, Aprison MH. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967;214:681–3. - PubMed
-
- Curtis DR, Hosli L, Johnston GA, Johnston IH. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5:235–58. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

