Clinical Development of a Cytomegalovirus DNA Vaccine: From Product Concept to Pivotal Phase 3 Trial
- PMID: 26344340
- PMCID: PMC4494211
- DOI: 10.3390/vaccines1040398
Clinical Development of a Cytomegalovirus DNA Vaccine: From Product Concept to Pivotal Phase 3 Trial
Abstract
2013 marks a milestone year for plasmid DNA vaccine development as a first-in-class cytomegalovirus (CMV) DNA vaccine enters pivotal phase 3 testing. This vaccine consists of two plasmids expressing CMV antigens glycoprotein B (gB) and phosphoprotein 65 (pp65) formulated with a CRL1005 poloxamer and benzalkonium chloride (BAK) delivery system designed to enhance plasmid expression. The vaccine's planned initial indication under investigation is for prevention of CMV reactivation in CMV-seropositive (CMV⁺) recipients of an allogeneic hematopoietic stem cell transplant (HCT). A randomized, double-blind placebo-controlled phase 2 proof-of-concept study provided initial evidence of the safety of this product in CMV⁺ HCT recipients who underwent immune ablation conditioning regimens. This study revealed a significant reduction in viral load endpoints and increased frequencies of pp65-specific interferon-γ-producing T cells in vaccine recipients compared to placebo recipients. The results of this endpoint-defining trial provided the basis for defining the primary and secondary endpoints of a global phase 3 trial in HCT recipients. A case study is presented here describing the development history of this vaccine from product concept to initiation of the phase 3 trial.
Keywords: CMV end organ disease (EOD); benzalkonium chloride (BAK); cytomegalovirus (CMV); glycoprotein B (gB); hematopoietic cell transplant (HCT); phosphoprotein 65 (pp65); plasmid DNA vaccine; poloxamer CRL1005.
Figures
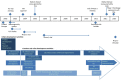
References
-
- Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. - PubMed
-
- Ulmer J.B., Donnelly J.J., Parker S.E., Rhodes G.H., Felgner P.L., Dwarki V.J., Gromkowski S.H., Deck R.R., DeWitt C.M., Friedman A., et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. - PubMed
-
- MacGregor R.R., Boyer J.D., Ugen K.E., Lacy K.E., Gluckman S.J., Bagarazzi M.L., Chattergoon M.A., Baine Y., Higgins T.J., Ciccarelli R.B., et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

