DNA-Encoded Flagellin Activates Toll-Like Receptor 5 (TLR5), Nod-like Receptor Family CARD Domain-Containing Protein 4 (NRLC4), and Acts as an Epidermal, Systemic, and Mucosal-Adjuvant
- PMID: 26344341
- PMCID: PMC4494208
- DOI: 10.3390/vaccines1040415
DNA-Encoded Flagellin Activates Toll-Like Receptor 5 (TLR5), Nod-like Receptor Family CARD Domain-Containing Protein 4 (NRLC4), and Acts as an Epidermal, Systemic, and Mucosal-Adjuvant
Abstract
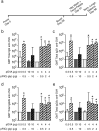
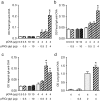
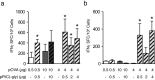
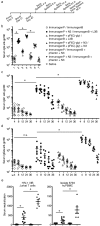
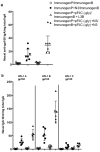
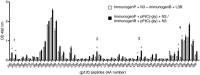
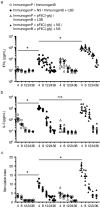
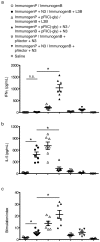
Eliciting effective immune responses using non-living/replicating DNA vaccines is a significant challenge. We have previously shown that ballistic dermal plasmid DNA-encoded flagellin (FliC) promotes humoral as well as cellular immunity to co-delivered antigens. Here, we observe that a plasmid encoding secreted FliC (pFliC(-gly)) produces flagellin capable of activating two innate immune receptors known to detect flagellin; Toll-like Receptor 5 (TLR5) and Nod-like Receptor family CARD domain-containing protein 4 (NRLC4). To test the ability of pFliC(-gly) to act as an adjuvant we immunized mice with plasmid encoding secreted FliC (pFliC(-gly)) and plasmid encoding a model antigen (ovalbumin) by three different immunization routes representative of dermal, systemic, and mucosal tissues. By all three routes we observed increases in antigen-specific antibodies in serum as well as MHC Class I-dependent cellular immune responses when pFliC(-gly) adjuvant was added. Additionally, we were able to induce mucosal antibody responses and Class II-dependent cellular immune responses after mucosal vaccination with pFliC(-gly). Humoral immune responses elicited by heterologus prime-boost immunization with a plasmid encoding HIV-1 from gp160 followed by protein boosting could be enhanced by use of pFliC(-gly). We also observed enhancement of cross-clade reactive IgA as well as a broadening of B cell epitope reactivity. These observations indicate that plasmid-encoded secreted flagellin can activate multiple innate immune responses and function as an adjuvant to non-living/replicating DNA immunizations. Moreover, the capacity to elicit mucosal immune responses, in addition to dermal and systemic properties, demonstrates the potential of flagellin to be used with vaccines designed to be delivered by various routes.
Keywords: DNA adjuvant; NLRC4; TLR5; adaptive immunity; flagellin.
Figures

References
-
- Jones S., Evans K., McElwaine-Johnn H., Sharpe M., Oxford J., Lambkin-Williams R., Mant T., Nolan A., Zambon M., Ellis J., et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine. 2009;27:2506–2512. doi: 10.1016/j.vaccine.2009.02.061. - DOI - PubMed
-
- Applequist S.E., Rollman E., Wareing M.D., Liden M., Rozell B., Hinkula J., Ljunggren H.G. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J. Immunol. 2005;175:3882–3891. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

