Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells
- PMID: 26344365
- PMCID: PMC5003084
- DOI: 10.1016/j.biomaterials.2015.08.048
Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells
Abstract
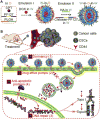
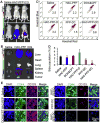
Dual responsive nanoparticles are developed for co-delivery of multiple anticancer drugs to target the drug resistance mechanisms of cancer stem-like cells (CSCs). The nanoparticles consist of four polymers approved by the Food and Drug Administration (FDA) for medical use: Poly(d,l-lactide-co-glycolide) (PLGA), Pluronic F127 (PF127), chitosan, and hyaluronic acid (HA). By combining PLGA and PF127 together, more stable and uniform-sized nanoparticles can be obtained than using PLGA or PF127 alone. The HA is used for not only actively targeting CSCs to reduce their drug resistance due to dormancy (i.e., slow metabolism), but also replacing the commonly used poly(vinyl alcohol) as a stabilizing agent to synthesize the nanoparticles using the double-emulsion approach and to allow for acidic pH-triggered drug release and thermal responsiveness. Besides minimizing drug efflux from CSCs, the nanoparticles encapsulated with doxorubicin hydrochloride (DOX, hydrophilic) and irinotecan (CPT, hydrophobic) to inhibit the activity of topoisomerases II and I, respectively, can fight against the CSC drug resistance associated with their enhanced DNA repair and anti-apoptosis. Ultimately, the two drugs-laden nanoparticles can be used to efficiently destroy the CSCs both in vitro and in vivo with up to ∼500 times of enhancement compared to the simple mixture of the two drugs.
Keywords: CD44; Cancer stem-like cell; Co-delivery; Drug resistance; Topoisomerase.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

