Adjuvants in the Driver's Seat: How Magnitude, Type, Fine Specificity and Longevity of Immune Responses Are Driven by Distinct Classes of Immune Potentiators
- PMID: 26344620
- PMCID: PMC4494256
- DOI: 10.3390/vaccines2020252
Adjuvants in the Driver's Seat: How Magnitude, Type, Fine Specificity and Longevity of Immune Responses Are Driven by Distinct Classes of Immune Potentiators
Abstract
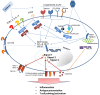
The mechanism by which vaccine adjuvants enhance immune responses has historically been considered to be the creation of an antigen depot. From here, the antigen is slowly released and provided to immune cells over an extended period of time. This "depot" was formed by associating the antigen with substances able to persist at the injection site, such as aluminum salts or emulsions. The identification of Pathogen-Associated Molecular Patterns (PAMPs) has greatly advanced our understanding of how adjuvants work beyond the simple concept of extended antigen release and has accelerated the development of novel adjuvants. This review focuses on the mode of action of different adjuvant classes in regards to the stimulation of specific immune cell subsets, the biasing of immune responses towards cellular or humoral immune response, the ability to mediate epitope spreading and the induction of persistent immunological memory. A better understanding of how particular adjuvants mediate their biological effects will eventually allow them to be selected for specific vaccines in a targeted and rational manner.
Keywords: Th1; Th17; Th2; adjuvant; immune epitope; immune mechanism; infectious disease; mucosal immunity; vaccine.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

