Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands
- PMID: 26344622
- PMCID: PMC4494261
- DOI: 10.3390/vaccines2020323
Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands
Abstract
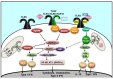
Vaccine efficacy is optimized by addition of immune adjuvants. However, although adjuvants have been used for over a century, to date, only few adjuvants are approved for human use, mostly aimed at improving vaccine efficacy and antigen-specific protective antibody production. The mechanism of action of immune adjuvants is diverse, depending on their chemical and molecular nature, ranging from non-specific effects (i.e., antigen depot at the immunization site) to specific activation of immune cells leading to improved host innate and adaptive responses. Although the detailed molecular mechanism of action of many adjuvants is still elusive, the discovery of Toll-like receptors (TLRs) has provided new critical information on immunostimulatory effect of numerous bacterial components that engage TLRs. These ligands have been shown to improve both the quality and the quantity of host adaptive immune responses when used in vaccine formulations targeted to infectious diseases and cancer that require both humoral and cell-mediated immunity. The potential of such TLR adjuvants in improving the design and the outcomes of several vaccines is continuously evolving, as new agonists are discovered and tested in experimental and clinical models of vaccination. In this review, a summary of the recent progress in development of TLR adjuvants is presented.
Keywords: TLR; cancer; immune response; microbial pathogens; vaccine adjuvant.
Figures
References
-
- Glenny A.T. Immunological notes: Antigenic value of toxoid precipitated by potassium alum. J. Pathol. Bacteriol. 1926;29:38–39.
-
- Freund J. The mode of action of immunologic adjuvants. Bibl. Tuberc. 1956;10:130–148. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

