MRI of rod cell compartment-specific function in disease and treatment in vivo
- PMID: 26344734
- PMCID: PMC8091657
- DOI: 10.1016/j.preteyeres.2015.09.001
MRI of rod cell compartment-specific function in disease and treatment in vivo
Abstract
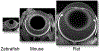
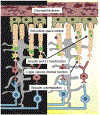
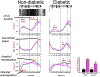
Rod cell oxidative stress is a major pathogenic factor in retinal disease, such as diabetic retinopathy (DR) and retinitis pigmentosa (RP). Personalized, non-destructive, and targeted treatment for these diseases remains elusive since current imaging methods cannot analytically measure treatment efficacy against rod cell compartment-specific oxidative stress in vivo. Over the last decade, novel MRI-based approaches that address this technology gap have been developed. This review summarizes progress in the development of MRI since 2006 that enables earlier evaluation of the impact of disease on rod cell compartment-specific function and the efficacy of anti-oxidant treatment than is currently possible with other methods. Most of the new assays of rod cell compartment-specific function are based on endogenous contrast mechanisms, and this is expected to facilitate their translation into patients with DR and RP, and other oxidative stress-based retinal diseases.
Keywords: Animal models; Calcium channels; Diabetes; MRI; Retinitis pigmentosa; Retinopathy; Subretinal space; Vision.
Copyright © 2015. Published by Elsevier Ltd.
Figures
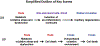
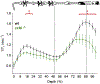

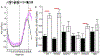
References
-
- Alder VA, Yu DY, Cringle SJ, Su EN, 1991. Changes in vitreal oxygen tension distribution in the streptozotocin diabetic rat. Diabetologia 34, 469–476. - PubMed
-
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, 1998. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 47, 1953–1959. - PubMed
-
- Arteel GE, Thurman RG, Raleigh JA, 1998. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur. J. Biochem 253, 743–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

