Peptide Dose and/or Structure in Vaccines as a Determinant of T Cell Responses
- PMID: 26344744
- PMCID: PMC4494221
- DOI: 10.3390/vaccines2030537
Peptide Dose and/or Structure in Vaccines as a Determinant of T Cell Responses
Abstract
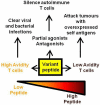
While T cells recognise the complex of peptide and major histocompatibility complex (MHC) at the cell surface, changes in the dose and/or structure of the peptide component can have profound effects on T cell activation and function. In addition, the repertoire of T cells capable of responding to any given peptide is variable, but broader than a single clone. Consequently, peptide parameters that affect the interaction between T cells and peptide/MHC have been shown to select particular T cell clones for expansion and this impacts on clearance of disease. T cells with high functional avidity are selected on low doses of peptide, while low avidity T cells are favoured in high peptide concentrations. Altering the structure of the peptide ligand can also influence the selection and function of peptide-specific T cell clones. In this review, we will explore the evidence that the choice of peptide dose or the structure of the peptide are critical parameters in an effective vaccine designed to activate T cells.
Keywords: T cell avidity; altered peptide ligands; functional avidity; peptide dose; peptide structure; peptide vaccines; vaccination.
Figures
References
-
- Bachmann M.F., Kalinke U., Althage A., Freer G., Burkhart C., Roost H., Aguet M., Hengartner H., Zinkernagel R.M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials