A Systematic Review of Recent Advances in Equine Influenza Vaccination
- PMID: 26344892
- PMCID: PMC4494246
- DOI: 10.3390/vaccines2040797
A Systematic Review of Recent Advances in Equine Influenza Vaccination
Abstract
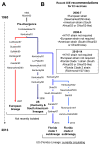
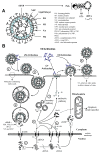
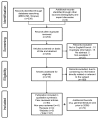
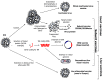
Equine influenza (EI) is a major respiratory disease of horses, which is still causing substantial outbreaks worldwide despite several decades of surveillance and prevention. Alongside quarantine procedures, vaccination is widely used to prevent or limit spread of the disease. The panel of EI vaccines commercially available is probably one of the most varied, including whole inactivated virus vaccines, Immuno-Stimulating Complex adjuvanted vaccines (ISCOM and ISCOM-Matrix), a live attenuated equine influenza virus (EIV) vaccine and a recombinant poxvirus-vectored vaccine. Several other strategies of vaccination are also evaluated. This systematic review reports the advances of EI vaccines during the last few years as well as some of the mechanisms behind the inefficient or sub-optimal response of horses to vaccination.
Keywords: equid; equine influenza; vaccine.
Figures
References
-
- Landolt G., Townsend H.G., Lunn D.P. Equine influenza infection. In: Sellon D.C., Long M.T., editors. Equine Infectious Diseases. Saunders Elsevier; St Louis, MO, USA: 2007. pp. 124–134.
-
- Wilson W.D. Equine influenza. Vet. Clin. North Am. Equine Pract. 1993;9:257–282. - PubMed
-
- Hannant D., Mumford J.A. Equine influenza. In: Studdert M.J., Horzinek M.C., editors. Virus Infections of Vertebrates. Elsevier Science Publishers; Amsterdam, The Netherlands: 1996. pp. 285–293. Volume Virus Infections of Equines.
-
- Miller W.C. Equine influenza. Further observations on the “Coughing” Outbreak, 1965. Vet. Rec. 1965;77:455–456. - PubMed
-
- Peek S.F., Landolt G., Karasin A.I., Slack J.A., Steinberg H., Semrad S.D., Olsen C.W. Acute respiratory distress syndrome and fatal interstitial pneumonia associated with equine influenza in a neonatal foal. J. Vet. Intern. Med. 2004;18:132–134. doi: 10.1111/j.1939-1676.2004.tb00149.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

