Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells
- PMID: 26344908
- PMCID: PMC4624935
- DOI: 10.1016/j.stemcr.2015.08.004
Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells
Abstract
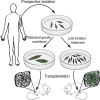
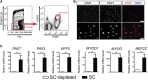
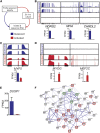
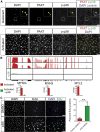
Adult skeletal muscle stem cells, or satellite cells (SCs), regenerate functional muscle following transplantation into injured or diseased tissue. To gain insight into human SC (huSC) biology, we analyzed transcriptome dynamics by RNA sequencing of prospectively isolated quiescent and activated huSCs. This analysis indicated that huSCs differentiate and lose proliferative potential when maintained in high-mitogen conditions ex vivo. Further analysis of gene expression revealed that p38 MAPK acts in a transcriptional network underlying huSC self-renewal. Activation of p38 signaling correlated with huSC differentiation, while inhibition of p38 reversibly prevented differentiation, enabling expansion of huSCs. When transplanted, expanded huSCs differentiated to generate chimeric muscle and engrafted as SCs in the sublaminar niche with a greater frequency than freshly isolated cells or cells cultured without p38 inhibition. These studies indicate characteristics of the huSC transcriptome that promote expansion ex vivo to allow enhanced functional engraftment of a defined population of self-renewing huSCs.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Aristorena M., Blanco F.J., de Las Casas-Engel M., Ojeda-Fernandez L., Gallardo-Vara E., Corbi A., Botella L.M., Bernabeu C. Expression of endoglin isoforms in the myeloid lineage and their role during aging and macrophage polarization. J. Cell Sci. 2014;127:2723–2735. - PubMed
-
- Benjamin I.J., Guo Y., Srinivasan S., Boudina S., Taylor R.P., Rajasekaran N.S., Gottlieb R., Wawrousek E.F., Abel E.D., Bolli R. CRYAB and HSPB2 deficiency alters cardiac metabolism and paradoxically confers protection against myocardial ischemia in aging mice. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3201–H3209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

