Optimal Use of Vaccines for Control of Influenza A Virus in Swine
- PMID: 26344946
- PMCID: PMC4494241
- DOI: 10.3390/vaccines3010022
Optimal Use of Vaccines for Control of Influenza A Virus in Swine
Abstract
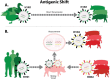
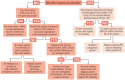
Influenza A virus in swine (IAV-S) is one of the most important infectious disease agents of swine in North America. In addition to the economic burden of IAV-S to the swine industry, the zoonotic potential of IAV-S sometimes leads to serious public health concerns. Adjuvanted, inactivated vaccines have been licensed in the United States for over 20 years, and there is also widespread usage of autogenous/custom IAV-S vaccines. Vaccination induces neutralizing antibodies and protection against infection with very similar strains. However, IAV-S strains are so diverse and prone to mutation that these vaccines often have disappointing efficacy in the field. This scientific review was developed to help veterinarians and others to identify the best available IAV-S vaccine for a particular infected herd. We describe key principles of IAV-S structure and replication, protective immunity, currently available vaccines, and vaccine technologies that show promise for the future. We discuss strategies to optimize the use of available IAV-S vaccines, based on information gathered from modern diagnostics and surveillance programs. Improvements in IAV-S immunization strategies, in both the short term and long term, will benefit swine health and productivity and potentially reduce risks to public health.
Keywords: immune response; influenza A virus in swine; surveillance; vaccines; veterinary diagnostics.
Figures


References
-
- Holtkamp D., Rotto H., Garcia R. The economic cost of major health challenges in large US swine production systems; Proceedings of the American Association of Swine Veterinarians Annual Meeting; Orlando, FL, USA. 3–6 March 2007.
-
- Donovan T.S. The role of influenza on growing pig performance; Proceedings of the Allen D. Leman Swine Conference; Saint Paul, MN, USA. 17–20 September 2005.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

