M2e-Based Universal Influenza A Vaccines
- PMID: 26344949
- PMCID: PMC4494237
- DOI: 10.3390/vaccines3010105
M2e-Based Universal Influenza A Vaccines
Abstract
The successful isolation of a human influenza virus in 1933 was soon followed by the first attempts to develop an influenza vaccine. Nowadays, vaccination is still the most effective method to prevent human influenza disease. However, licensed influenza vaccines offer protection against antigenically matching viruses, and the composition of these vaccines needs to be updated nearly every year. Vaccines that target conserved epitopes of influenza viruses would in principle not require such updating and would probably have a considerable positive impact on global human health in case of a pandemic outbreak. The extracellular domain of Matrix 2 (M2e) protein is an evolutionarily conserved region in influenza A viruses and a promising epitope for designing a universal influenza vaccine. Here we review the seminal and recent studies that focused on M2e as a vaccine antigen. We address the mechanism of action and the clinical development of M2e-vaccines. Finally, we try to foresee how M2e-based vaccines could be implemented clinically in the future.
Keywords: influenza; matrix protein 2; vaccines.
Figures


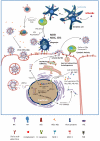
References
-
- CDC Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 2010;59:1057–1062. - PubMed
-
- Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A., Simmerman J.M., Gordon A., Sato M., Howie S., et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. - DOI - PubMed
-
- Patterson K.D., Pyle G.F. The geography and mortality of the 1918 influenza pandemic. Bull. Hist. Med. 1991;65:4–21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

